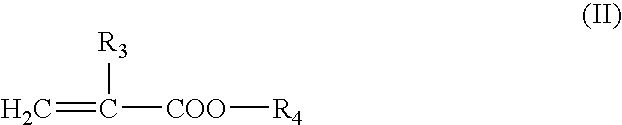Crosslinking polymer-supported porous film for battery separator and method for producing battery using the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
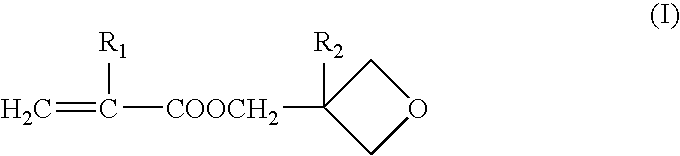
[0076] Production of 3-oxetanyl Group-Containing Crosslinking Polymer A (Weight Average Molecular Weight: 518,000 and Content of 3-oxetanyl Group-Containing Monomer Content: 25% by Weight)
[0077] 60.0 g of methyl methacrylate, 20.0 g of 3-ethyl-3-oxetanylmethyl methacrylate, 158.0 g of ethyl acetate and 0.16 g of N,N'-azobisisobutyronitrile were placed in a 500 ml three-necked flask equipped with a reflux condenser, and mixed for 30 minutes under stirring while introducing nitrogen gas. Radical polymerization was initiated at 60.degree. C. When about 2 hours passed, the viscosity of the reaction mixture began to increase. The reaction mixture was further polymerized for additional 8 hours. The reaction mixture was cooled to about 10.degree. C., and 0.16 g of azobisbutyronitrile was added thereto. The resulting mixture was again heated to 70.degree. C., and polymerization was conducted for 8 hours.
[0078] After completion of the reaction, the reaction mixture was cooled to about 40.deg...
production example 2
[0080] Production of 3-oxetanyl Group-Containing Crosslinking Polymer B (Weight Average Molecular Weight: 253,000 and Content of 3-oxetanyl Group-Containing Monomer Component: 15% by Weight)
[0081] In the same manner as in the Production Example 1, 68.0 g of methyl methacrylate, 12.0 g of 3-ethyl-3-oxetanylmethyl methacrylate, 158.0 g of ethyl acetate and 0.15 g of N,N'-azobisisobutyronitrile were placed in a 500 ml three-necked flask equipped with a reflux condenser, and mixed for 30 minutes under stirring while introducing nitrogen gas. Radical polymerization was initiated at 70.degree. C. When about 1.5 hours passed, the viscosity of the reaction mixture began to increase. The reaction mixture was further polymerized for additional 8 hours. The reaction mixture was cooled to about 40.degree. C., and 0.15 g of azobisbutyronitrile was added thereto. The resulting mixture was again heated to 70.degree. C., and polymerization was conducted for 8 hours.
[0082] After completion of the re...
production example 3
[0084] Production of 3-oxetanyl Group-Containing Crosslinking Polymer C (Weight Average Molecular Weight: 167,000 and Content of 3-oxetanyl Group-Containing Monomer Component: 40% by Weight)
[0085] 48.0 g of methyl methacrylate, 32.0 g of 3-ethyl-3-oxetanylmethyl methacrylate, 58.0 g of ethyl acetate and 0.36 g of N,N'-azobisisobutyronitrile were charged in a 500 ml three-neck flask equipped with a reflux condenser, for mixing were placed in a 500 ml three-necked flask equipped with a reflux condenser, and mixed for 30 minutes under stirring while introducing nitrogen gas, in the same manner as in the Production Example 1. Radical polymerization was initiated at 70.degree. C. When about 1.5 hours passed, the viscosity of the reaction mixture began to increase. The reaction mixture was further polymerized for additional 8 hours. The reaction mixture was cooled to about 40.degree. C., and 0.36 g of azobisbutyronitrile was added thereto. The resulting mixture was again heated to 70.degr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Porosity | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com