Oligodeoxynucleotide intervention for prevention and treatment of sepsis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
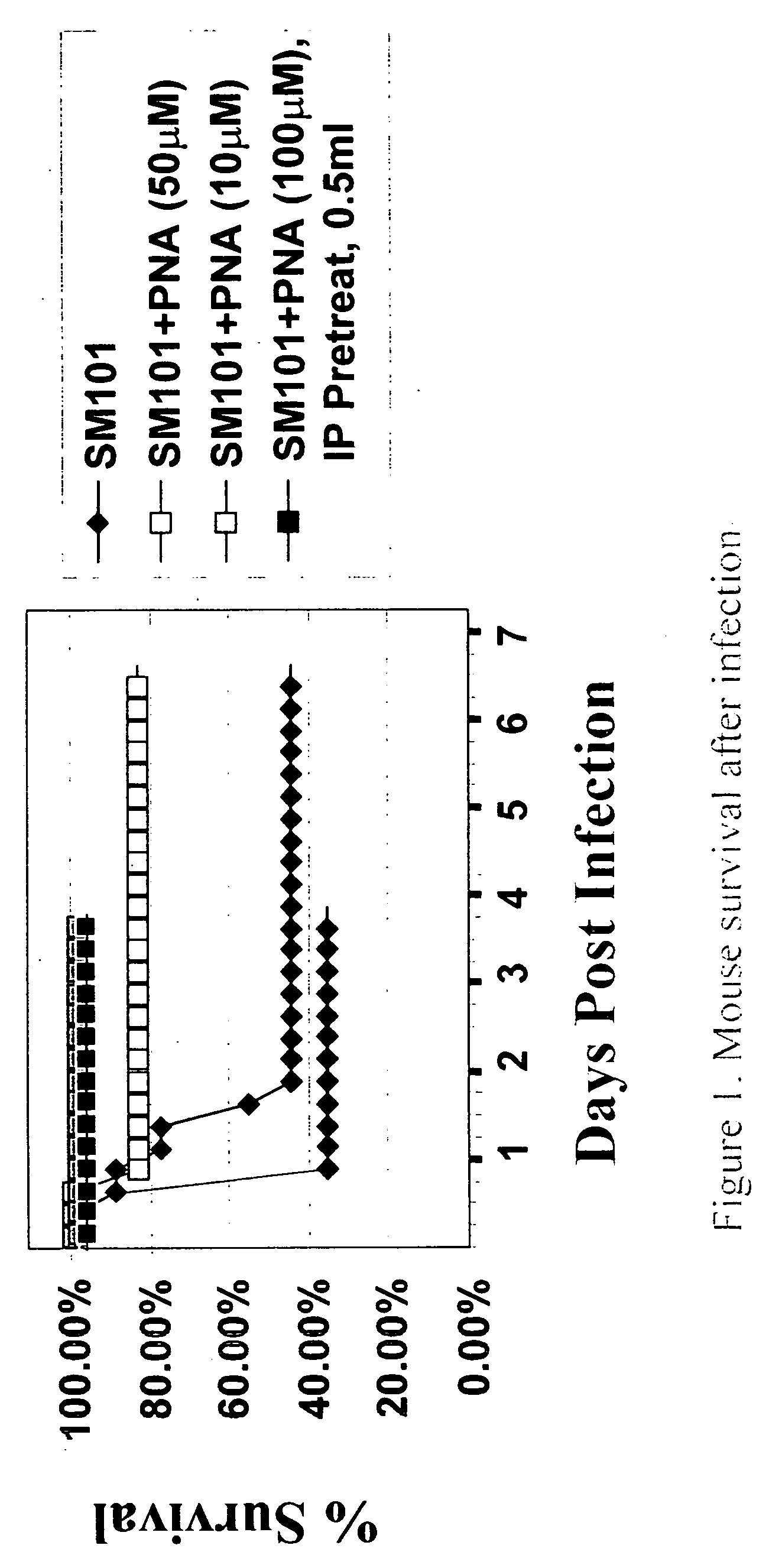
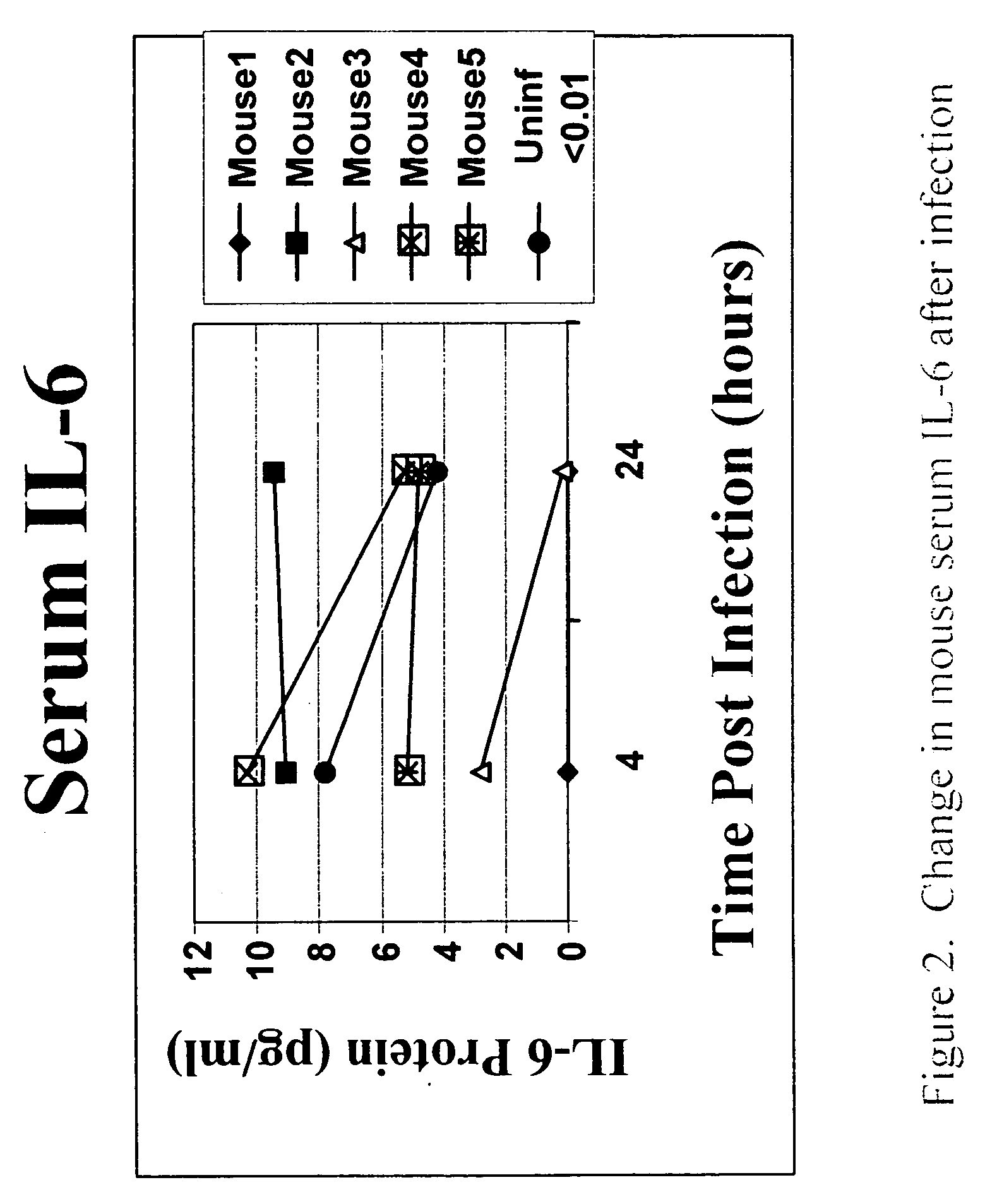
Development of the Mouse Sepsis Model
[0050]E. coli SM101, a temperature-sensitive UDP-N-acetylglucosamine acyltransferase mutant that lose all detectable acyltransferase activity, and its wild-type K12, were i.p. injected as described below to induce sepsis in mouse. SM101 has a defect in lipid A biosynthesis that causes the outer membrane to be permeable to high-molecular-weight substances. The lipid A content of SM101 is reduced 2-3-fold compared with the wild-type. To prepare the bacteria for mouse infection, SM101 were grown in LB medium at 37° C. Log-phase cultures of SM101 were grown to an optical density at 600 nm of 1.1 (equivalent to 5×108 CFU / ml), followed by centrifugation and resuspension in sterile phosphate-buffered saline (PBS) at 4° C. This log-phase preparation of bacteria was serially diluted in PBS, and 3×10e8 CFU of bacteria was i.p. injected to induce mouse sepsis. Serum was gathered and pro-inflammatory cytokines (IL-6, TNF, IL-1) and bacterial load tested, an...
example 2
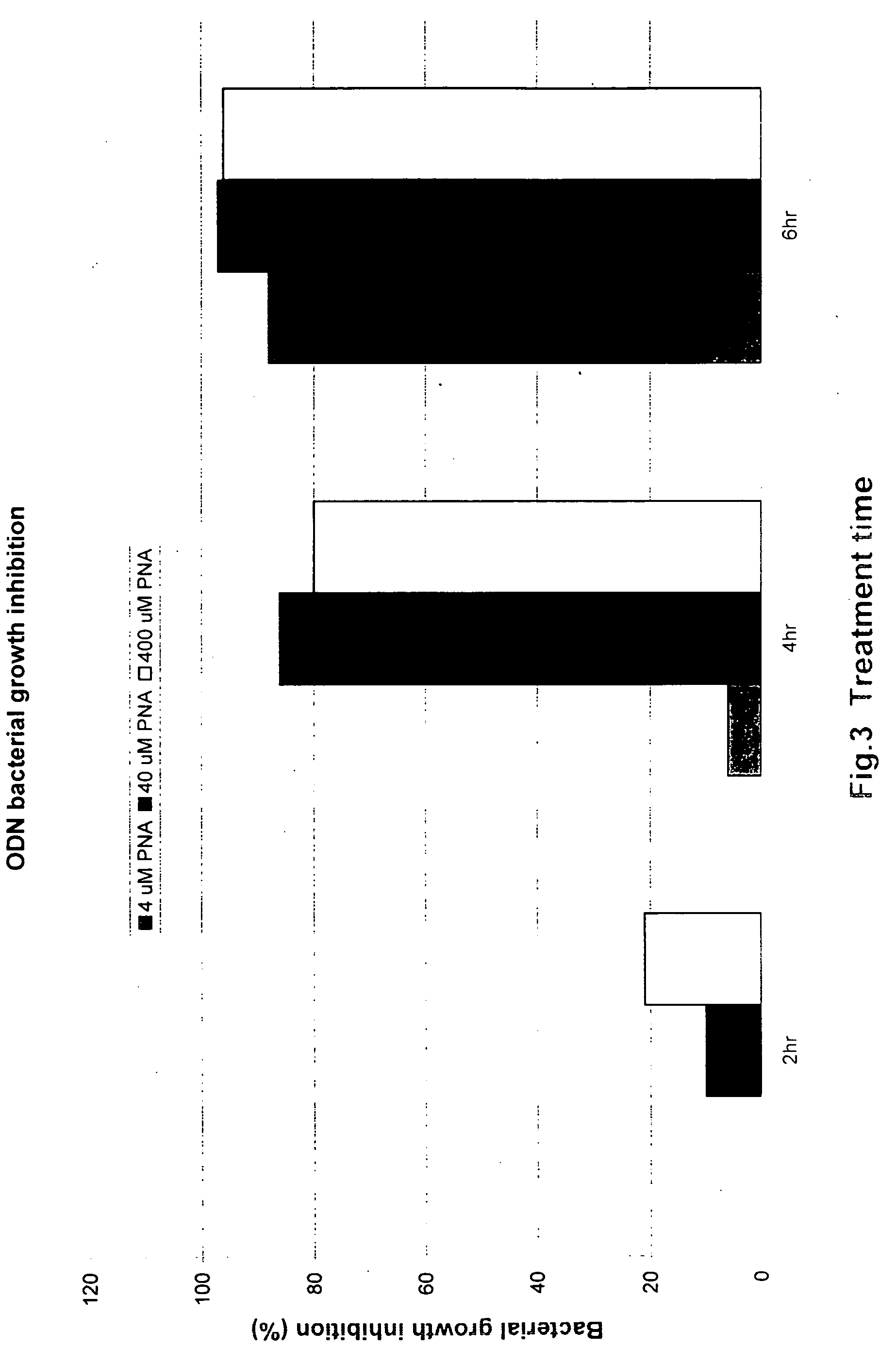
Inhibition of Bacterial Growth by ODN
[0051] The inhibition of bacterial growth by ODN was evaluated by examining the effect of DNA dose on the ability of ODN to inhibit SM101 growth. In this study, an ODN having the sequence CTC ATA CTC T was added to the 1 / 50 diluted O / N SM101 cell cultures, at final concentration of 40 μM or 400 μM, with addition of equal volume water as a negative control, and incubated with shaking at 30° C. After 2, 4 or 6 h, the growth was measured by either the optical density at 600 nm (OD600) or viable cell count, which was done by diluting the cultures and plating them in triplicate on LB plates with streptomycin. As shown in FIG. 3, upon addition of ODN, cell growth was inhibited by 86-96%.
example 3
Inhibition of Bacterial Growth by ODN Expression Plasmid
[0052] In this study, the ODN expression plasmid As080103, having the sequence CYGXO80103 listed above, and plasmid pssxGb without ODN insert as negative control, were transformed into E. coli XL10-gold(kan). The resulting cell cultures were plated on LB media with chloramphenicol and incubated at 37° C. O / N. As shown in FIG. 4, no XL10-gold(kan) carrying ODN expression plasmid grew on the LB media.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com