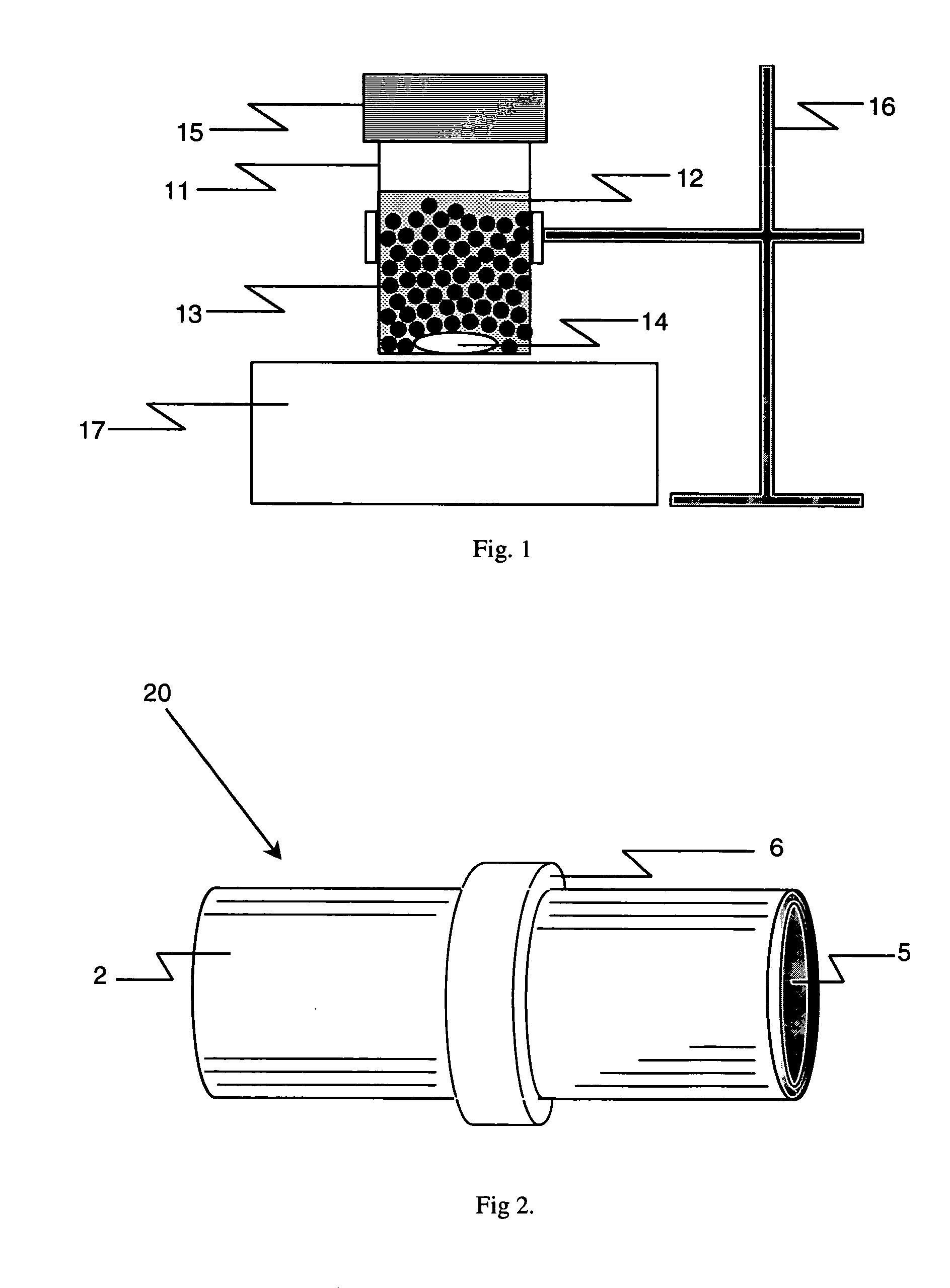
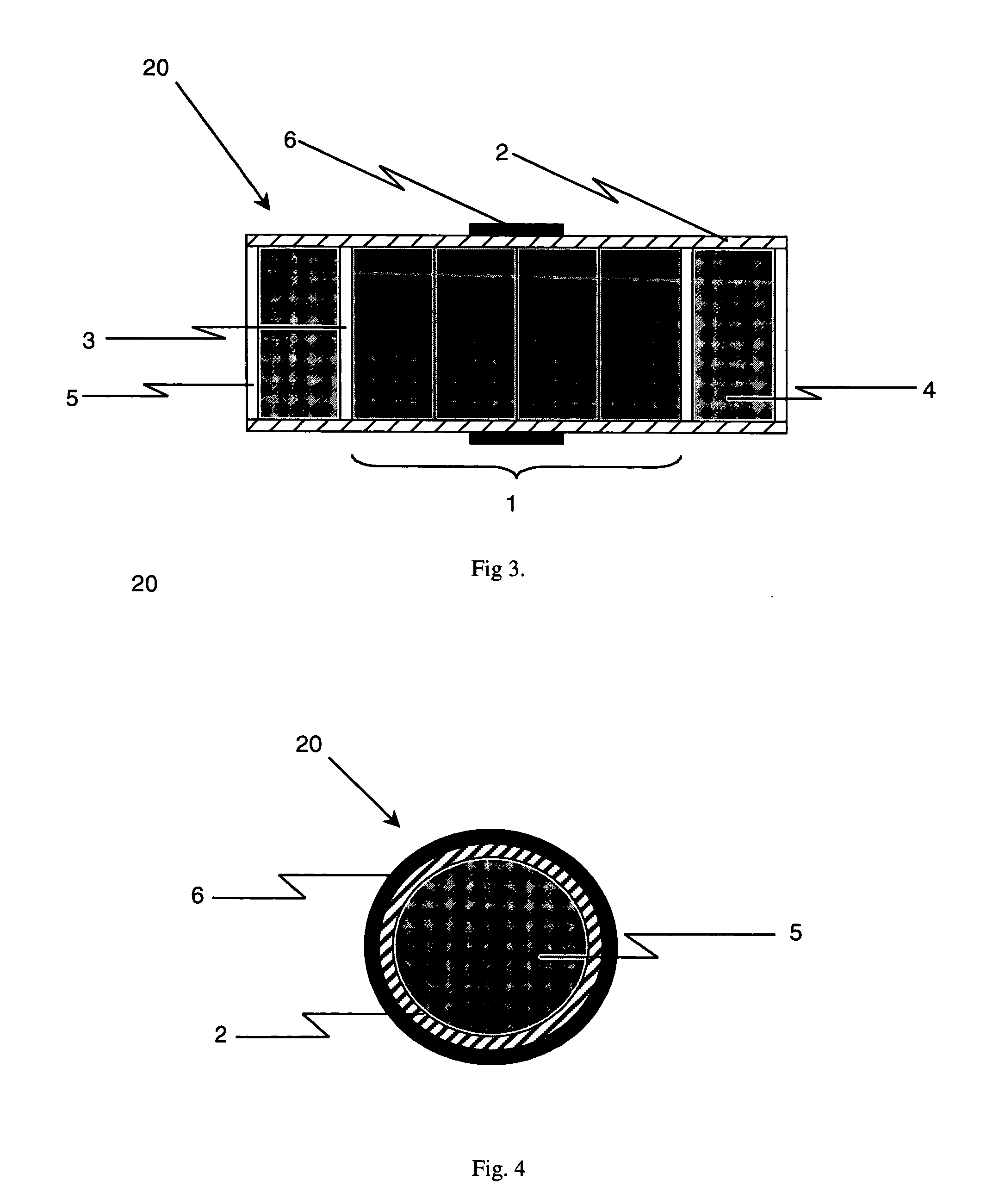
Laboratory scale milling process
a technology of laboratory scale and milling process, which is applied in the direction of centrifuges, instruments, and pharmaceutical product form changes, etc., can solve the problems preventing the use of known mill types in certain situations, and reducing the efficiency of drug manufacturing, so as to achieve the effect of reducing the particle size of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0101] Dispersions D1-D4 containing 5% by weight celecoxib were prepared by the process described below. The dispersions differed only in the particle size range of the celecoxib. [0102] 1. Celecoxib was micronized in an air jet mill to form a drug powder. [0103] 2. The drug powder was added to an aqueous solution containing 2.5% low viscosity hydroxypropylcellulose (HPC-SL) and 0.12% sodium dodecyl sulfate, to form a suspension. [0104] 3. The suspension was wet milled to form a milled suspension according to the following protocol. A sample amount of 6.0 ml of the suspension (containing 20% celecoxib), a 19 mm magnetic stir bar made by VWR, 8 ml of lead-free glass beads, and 50 μl of antifoaming agent (Sigma Antifoam A Concentrate) were added to a 20 ml scintillation vial. To provide a milled suspension having a target particle size range of 6-7 μm (i.e., the size range achieved in the micronizing step, used to provide a comparative composition), the vial was shaken for two minutes...
example 2
[0106] Celecoxib particle size in milled suspensions D1-D4 as prepared in Example 1 was determined by laser (Fraunhofer) diffraction and by optical microscopy.
[0107] Fraunhofer scattering was measured on static suspensions samples using a Sympatec spectrometer. Samples were diluted with water into a static cell at a concentration that maintained a reduction in laser intensity of approximately 20%. The choice of collection lens was determined by the population of large material present in suspension, and thus was different for each sample. However, the smallest focal length optic appropriate was used in each case. No Mie scattering corrections were applied. The results, presented in FIG. 5, show a D50 particle size consistent with the target size range. D50 and other particle size parameters shown in FIG. 5 are believed to be overestimated for the 0.2-0.4 μm celecoxib dispersion, since this size range is at the very limit of detection by this technique.
[0108] In order to visually c...
example 3
[0109] Samples of milled suspensions prepared in Example 1 were dried individually by rotovapping according to the following procedure: 3 ml of each 5% celecoxib suspension was placed in a 10 ml recovery flask and attached to a conventional laboratory roto-evaporator (Buchi, RE11 Rotovapor). The flask was suspended in water at a temperature of 40° C. and rotated at approximately 100 rpm. A gentle vacuum was pulled on the flask for 0.5-1 hour to avoid bubbling of the suspension. After this time, a full house vacuum was employed and the evaporation proceeded for not less than 48 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com