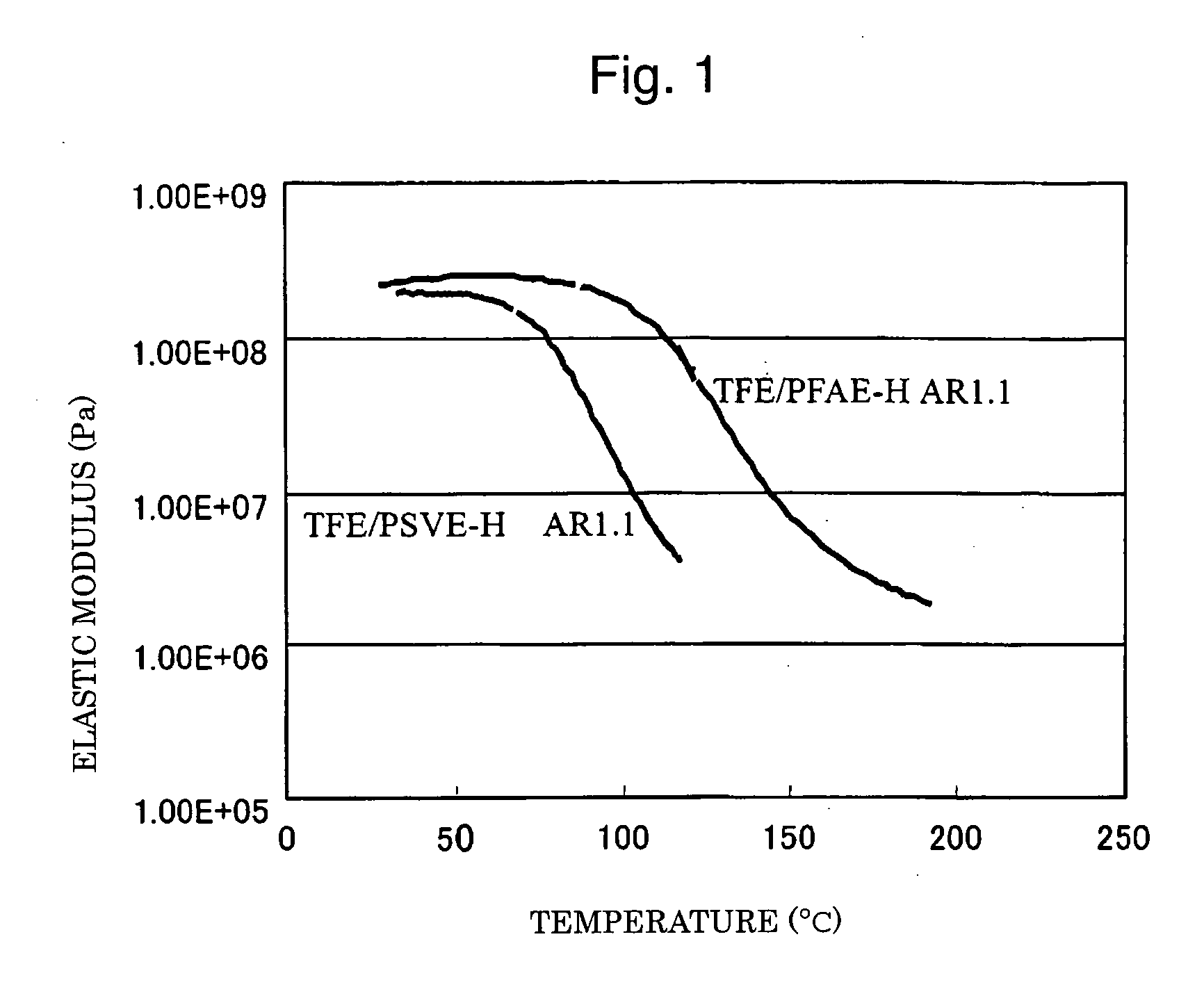
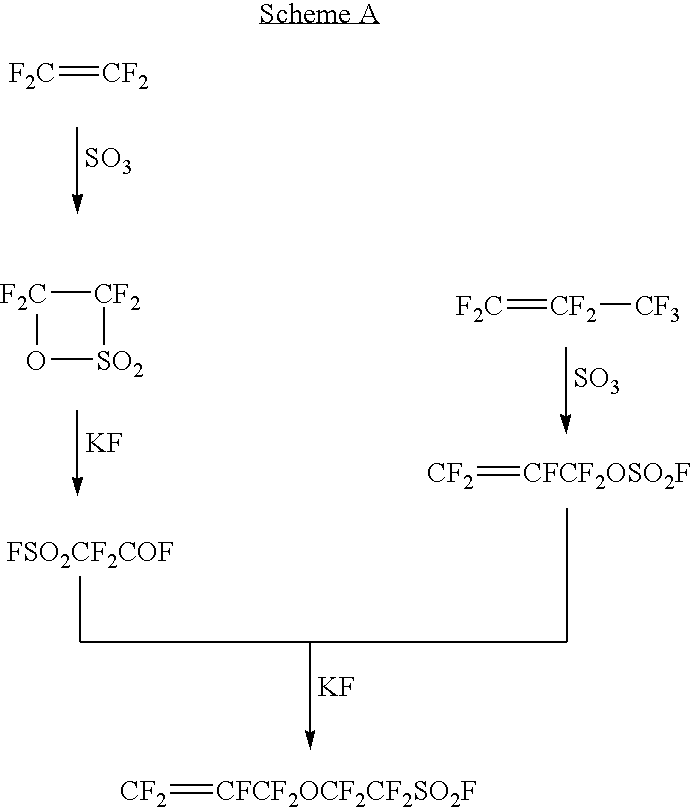
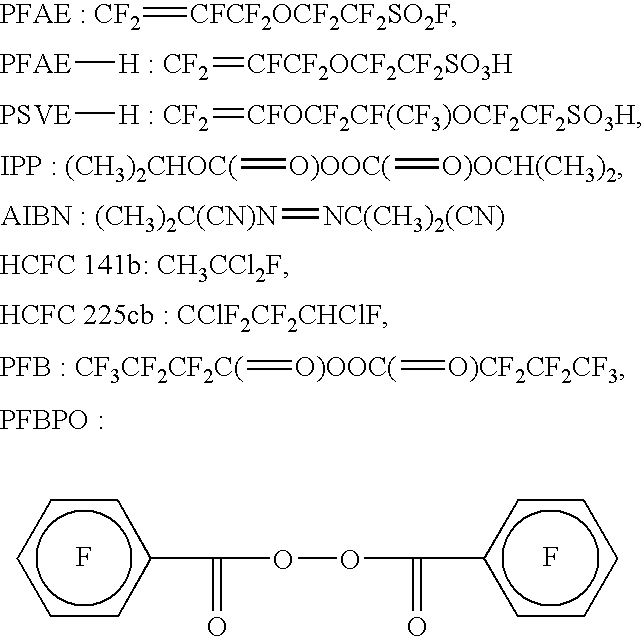
Polymer electrolyte fuel cell, electrolyte material therefore and method for its production
a fuel cell and electrolyte technology, applied in the direction of non-aqueous electrolyte cells, cell components, sustainable manufacturing/processing, etc., can solve the problems of insufficient ion exchange capacity of fuel cells, production cost was high, and production cost was not low, so as to achieve excellent ion conductivity and durability, and production cost can be substantially reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
POLYMERIZATION EXAMPLE 1
Into a stainless steel autoclave having an internal capacity of 125 cm3, 37.2 g of PFAE and, 205 mg of perfluorobenzoyl peroxide (PFBPO) as an initiator, were introduced and cooled with liquid nitrogen and deaerated. Then, TFE was introduced into the autoclave, and the system was maintained at 80° C. under 0.345 MPaG (gauge pressure, the same applies hereinafter) for 2 hours and 50 minutes. The autoclave was cooled, and the gas in the system was purged to stop the polymerization. After diluting with HCFC 225cb, the polymer was flocculated by an addition of HCFC 141b, followed by filtration. Then, the polymer was stirred in HCFC 225cb and re-flocculated by HCFC 141b, followed by vacuum drying overnight at 80° C. AR of the polymer obtained by titration was 1.12 meq / g, and TQ measured by means of Capillary Rheometer CFT-500D (manufactured by Shimadzu Corporation) was 204° C.
POLYMERIZATION EXAMPLES 2 to 5 and POLYMERIZATION REFERENCE EXAMPLES 1 and 2
TFE / PFAE ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| inner diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com