Novel agents for ameliorating motor disorder
a motor disorder and novel technology, applied in the field of medical drugs, can solve the problems of deteriorating physical function, difficulty in maintaining posture and position, and inability to walk or walk normally, and achieve the effect of meliorating dyskinesia and ameliorating dyskinesia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation 1
Purification of Selenoprotein P Fragment Using Anti-Selenoprotein P Fragment Antibody-Bound Carrier (Anti-SeP Antibody Column)
[0038] Heparin Sepharose-binding fraction from plasma was precipitated with 2 M ammonium sulfate. The precipitate was dissolved in more than 5 volumes of 20 mM Tris buffer, pH 8.0. Selenoprotein P present in this solution was adsorbed to anti-SeP antibody column and the carrier was washed with PBS. Selenoprotein P was eluted with 20 mM citrate buffer containing 4 M urea and was adsorbed to a cation exchanger (Macroprep High S: BioRad) equilibrated with 20 mM citrate buffer. Then, gradient elution was performed with a salt concentration of sodium chloride and a fraction of selenoprotein P fragment having the cell death-inhibitory activity was recovered. At this stage, a full-length selenoprotein P could also be obtained but with a cell death-inhibitory activity per proteins being much lower than that of the fragment thereof. According to the procedures as des...
example 1
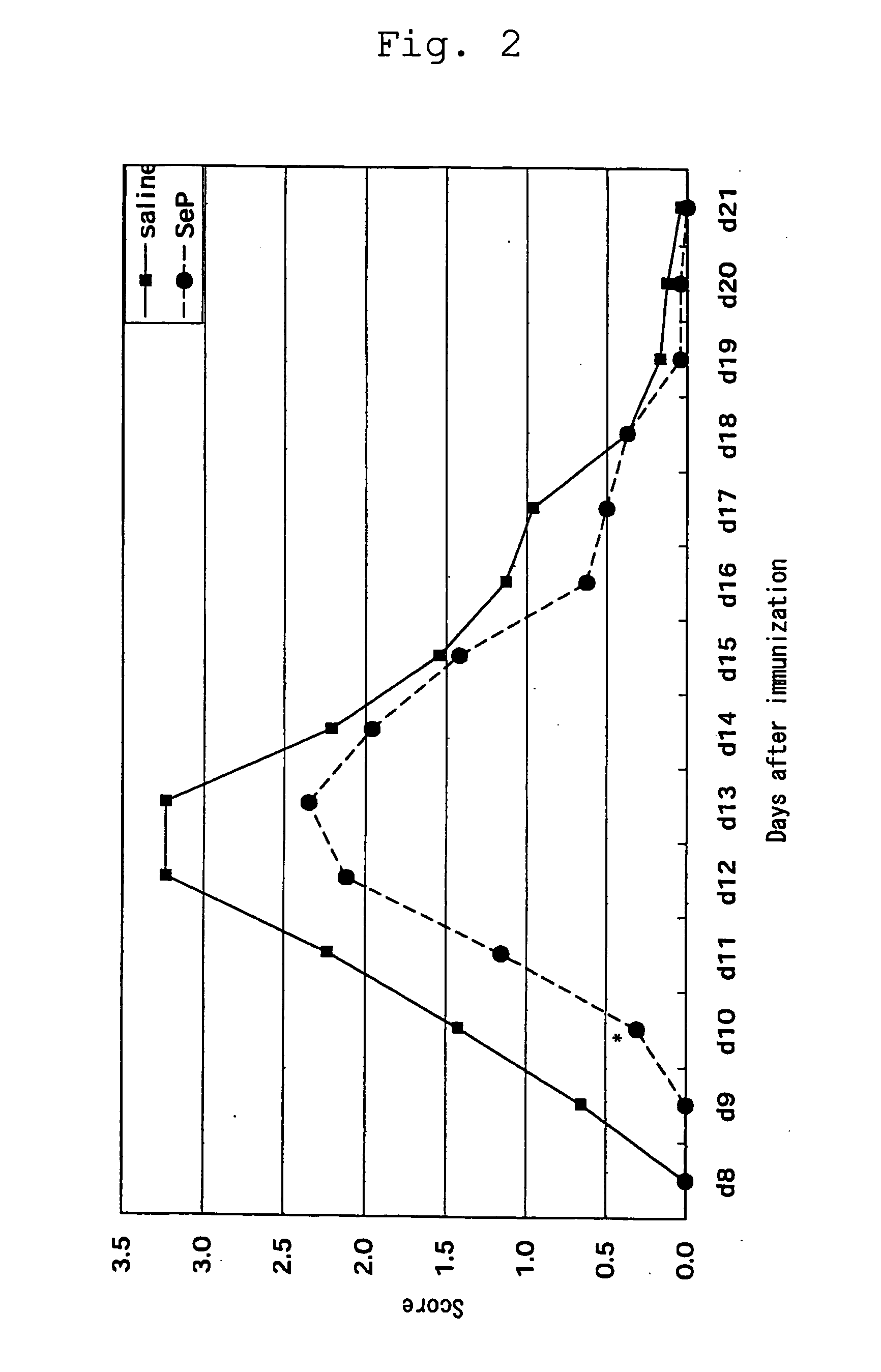
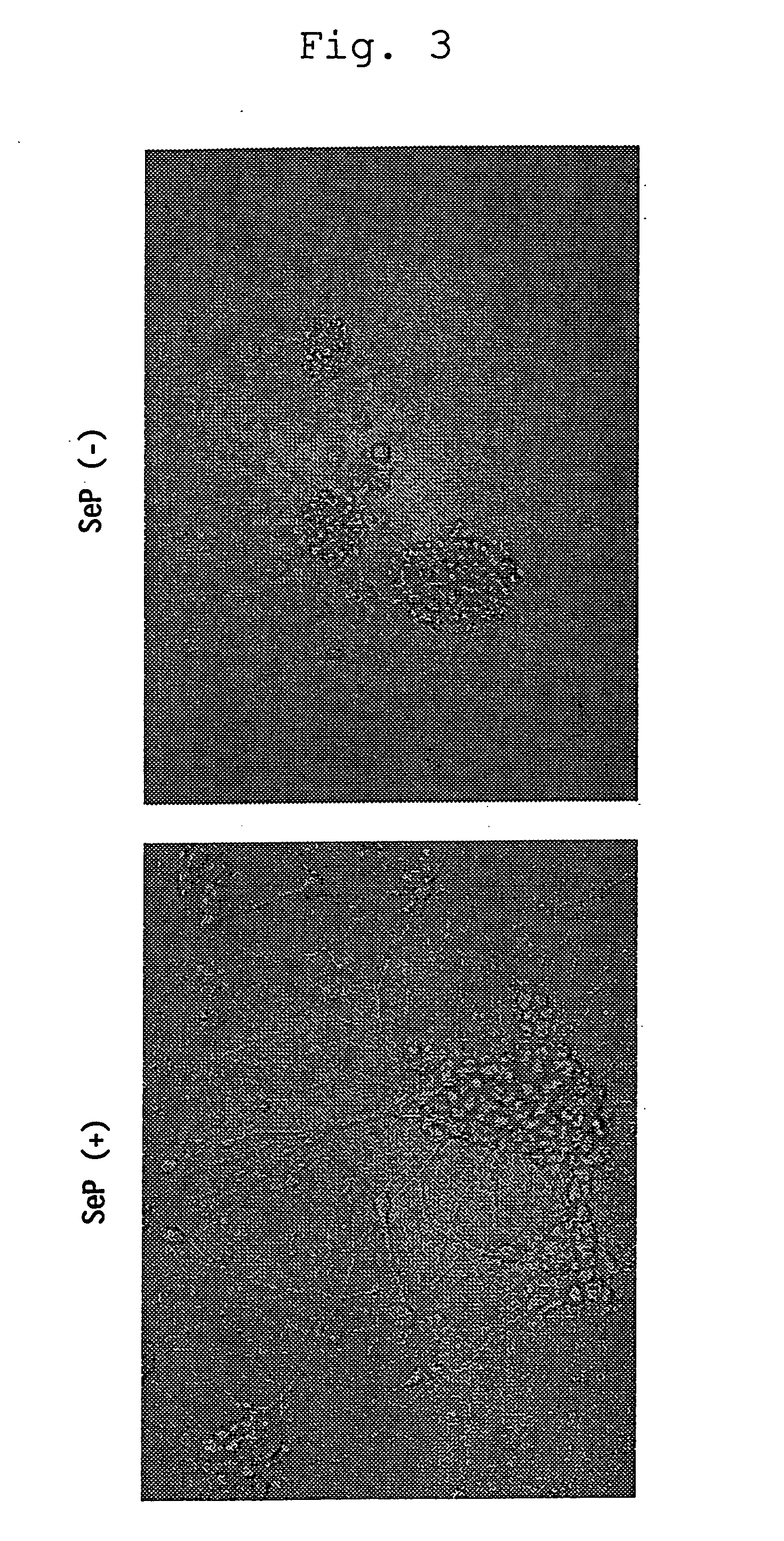
Cytotoxicity-Inhibitory Activity
[0039] Using Dami cells (described in Greenberg S. M. et al., Blood, vol. 72, p. 1968-1977 (1988)) for use in assay system for cytotoxicity-inhibitory activity, the cells were washed twice with assay medium (50% PBS / SA / 0.03% HSA (manufactured by SIGMA) or SA / 0.05% BSA free from fatty acid (WAKO) / 4 μM long-chain polyvalent fatty acid (e.g. arachidonic acid, linoleic acid or linolenic acid)) and suspended in the same medium at 3×104 cells / ml. The cell suspension was added to a 96-well plate in each 200 μl for wells for sample addition or in each 100 μl for wells for serial dilution. To the wells for sample addition was added each 2 μl assay sample containing either selenoprotein P fragment prepared in Preparation 1, selenocystine, selenomethionine, Ebselen, or sodium selenite at the same concentration. After stirring, a serial dilution was made with the wells containing 100 μl cell suspension. The plate was incubated at 37° C. in CO2 incubator for 4 to...
example 2
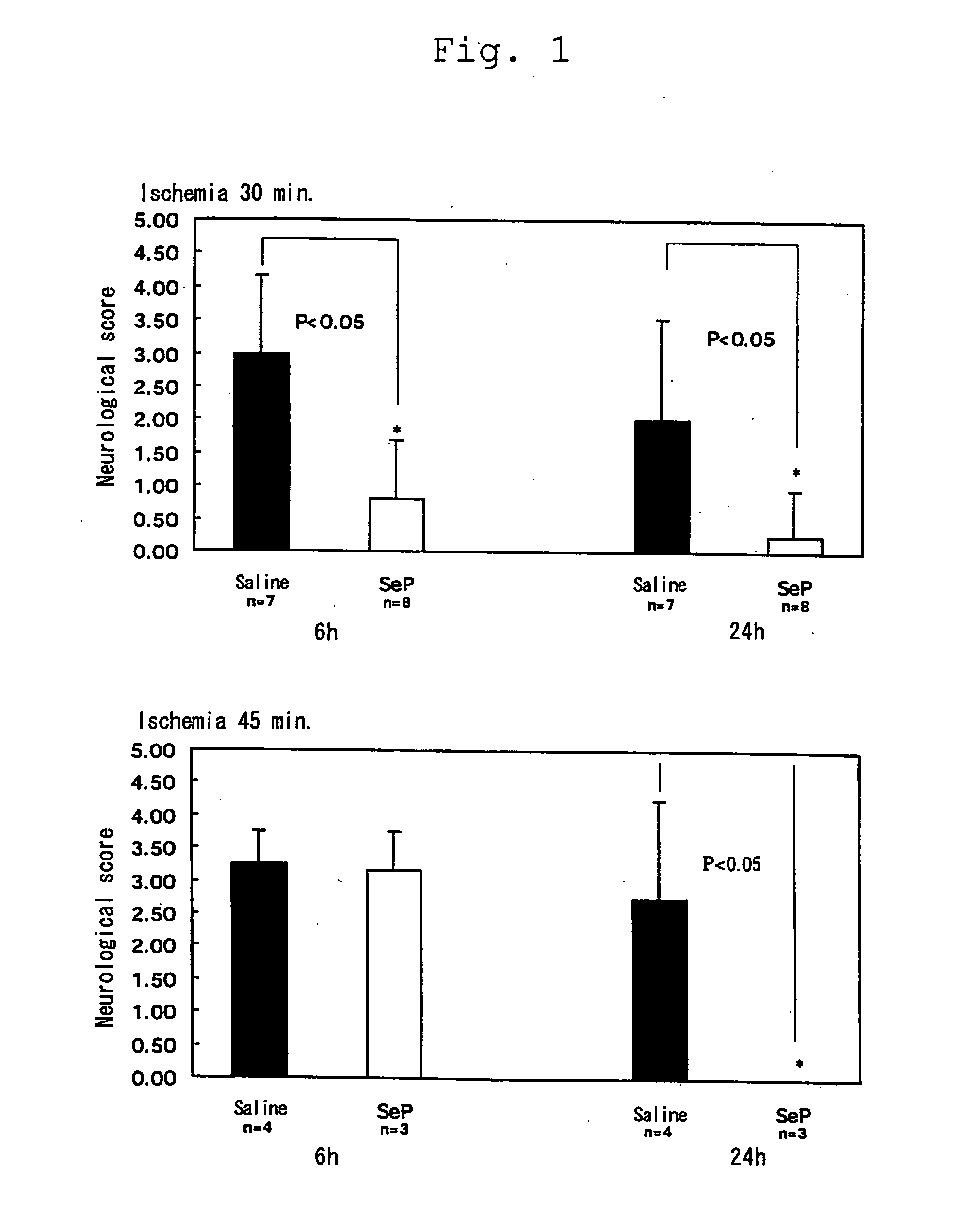
Inhibitory Effect of Selenoprotein P Fragment on Ischemia / Reperfusion Injury in Cerebral Ischemia / Reperfusion Injury Model
[0041] Effect of selenoprotein P on dyskinesia induced by cerebral ischemia / reperfusion injury was assessed with a degree of paralysis using gerbil mice of 12 weeks old. The animals were systemically anesthetized by intraperitoneal injection of ketamine hydrochloride (100 mg / kg), the cervical vein was revealed by midline incision, and received 1 mg / animal of selenoprotein P fragment of the present invention via intravenous administration. After ischemia for 30 minutes or 40 minutes, the bloodstream was recovered for reperfusion. A degree of paralysis was assessed after 6 and 24 hours.
[0042] A degree of paralysis after 6 and 24 hours was assessed in accordance with the score indicated in Table 1 below.
TABLE 1Normal0Light paralysis in the forefoot, dull movement1in a posture of a bent footA little worsened paralysis in the foot,2continuously turning round to on...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| oxidative stress | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com