A small molecular polypeptide and its application in the preparation of drugs for preventing and treating Parkinson's syndrome
A Parkinson's and drug technology, applied in the application field of small molecular peptides in the preparation of drugs for the prevention or treatment of Parkinson's syndrome, can solve the problems that patients can only be treated symptomatically, cannot be effectively cured, and have limited treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of TAT-pSyn
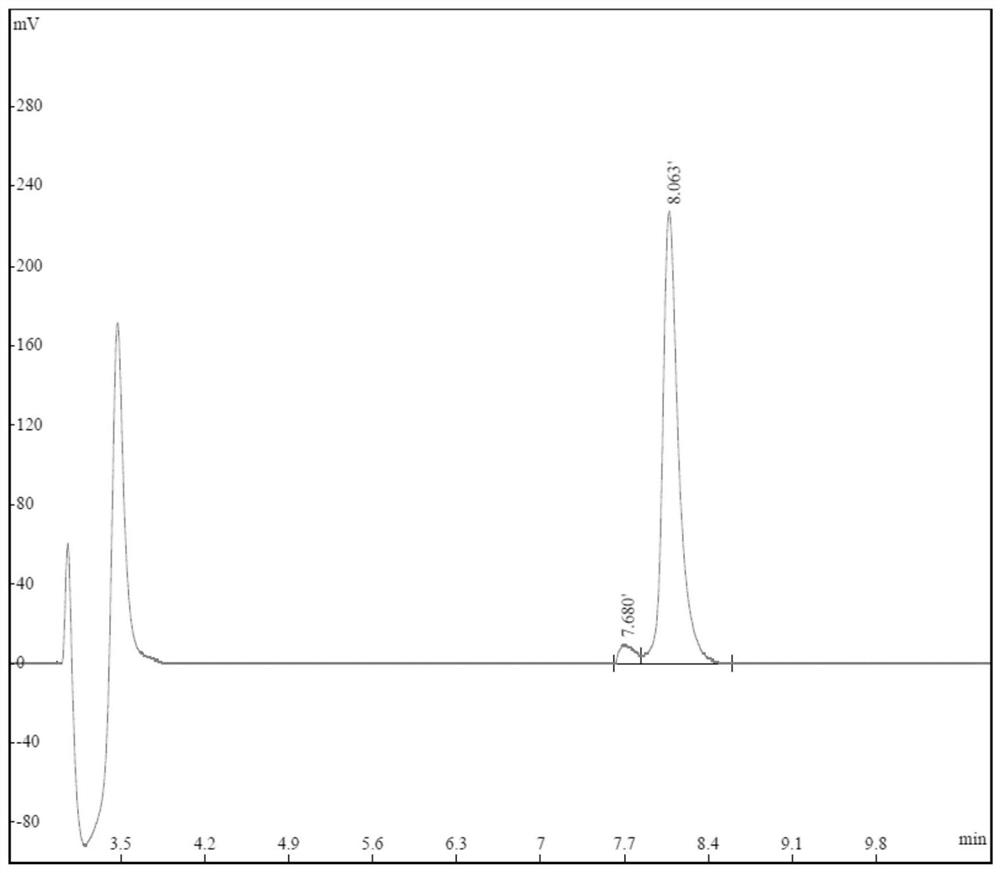
[0032] The sequence of TAT-pSyn is shown as SEQ ID NO.1, which was artificially synthesized by Jiangsu Qiangyao Biotechnology Co., Ltd. The synthesis report is as follows, and the chromatogram is as follows figure 1 shown.
[0033] TAT-pSyn synthetic HPLC report
[0034] Product name: 04010035452 Peptide name: TAT-pSyn Measurement: Peak Area Calculation Type: Percentage Injection volume: 10 microliters Passing time: 11 minutes Chromatographic column: Kromasil 100-5C18, 4.6mm*250mm, 5micron Flow rate: 1ml / min Solvent A: 0.1% TFA in water Column temperature: 25°C Solvent B: 0.1% TFA in Acetonitrile Wavelength: 220nm Solvent gradient: 15%-35% buffer B in 11min
[0035] wavelength time Peak area Peak Area Percentage (%) 1 220nm 7.680 92944 4.21 2 220nm 8.063 2114477 95.79 total 2207421 100
[0036] The purity of the synthesized TAT-pSyn poly...
Embodiment 2
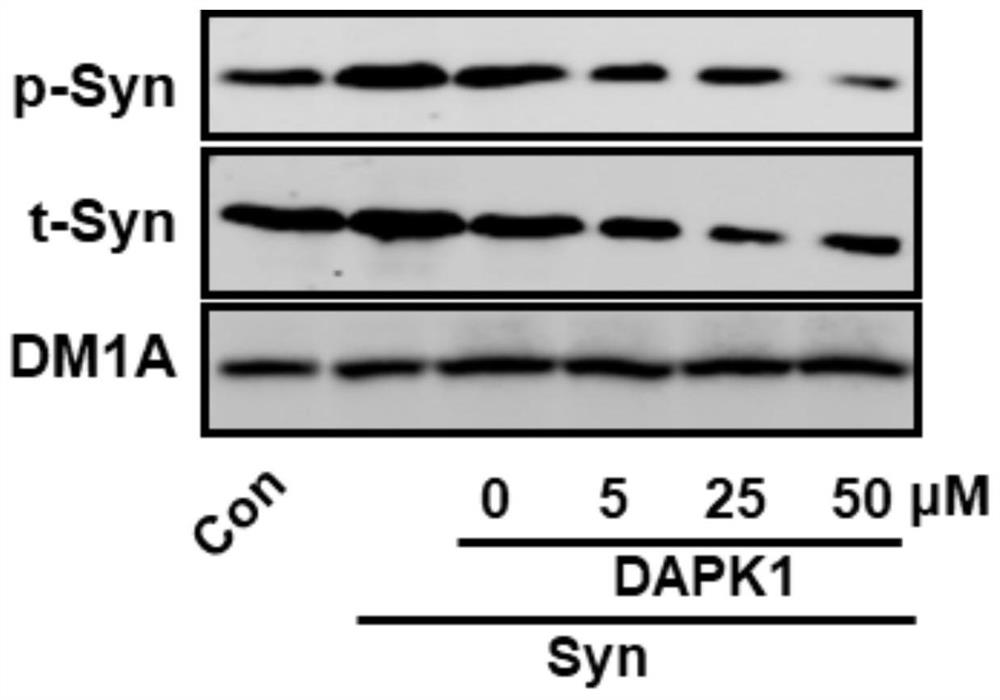
[0040] TAT-pSyn blocks the combination of DAPK1 and alpha-synuclein, and inhibits the hyperphosphorylation and abnormal aggregation of alpha-synuclein.
[0041] Culture neurofibroma blastocytes (N2a) in vitro, transfect eukaryotic expression plasmids of alpha-synuclein, and give 0uM, 5uM, 25uM, 50uM TAT-pSyn polypeptide or control TAT- s-pSyn polypeptide or vehicle incubation. Cellular proteins were extracted after 90 minutes. The result shows: after giving the TAT-pSyn of 25uM, the protein amount of alpha-synuclein and the alpha-synuclein of phosphorylation all reduce ( figure 2 ). It suggested that TAT-pSyn blocked the interaction between DAPK1 and alpha-synuclein, thereby inhibiting the hyperphosphorylation and abnormal aggregation of alpha-synuclein.
Embodiment 3
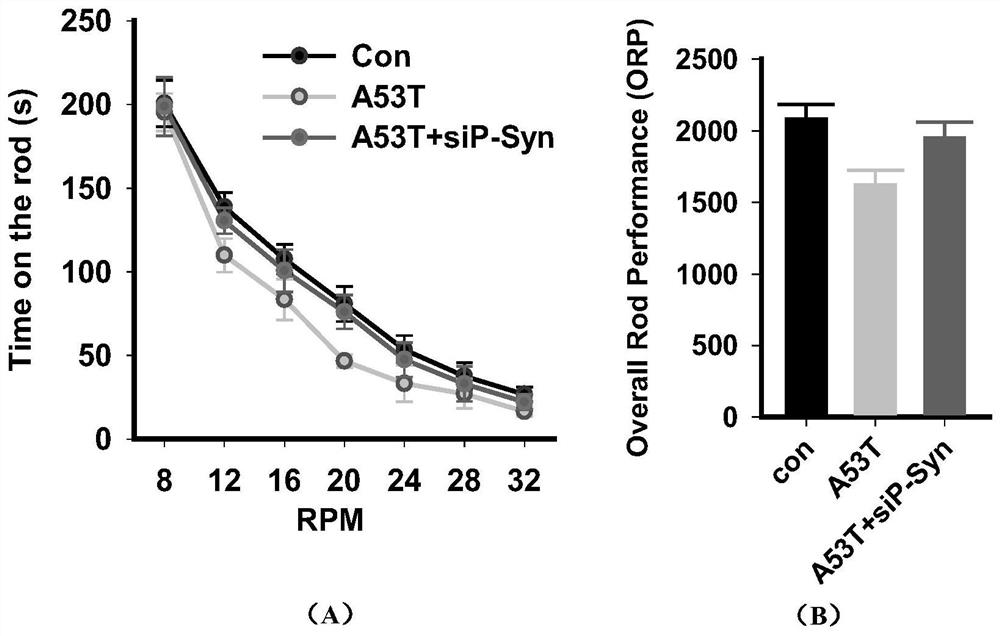
[0043] Application of TAT-pSyn in transgenic mouse model of Parkinson's syndrome
[0044] (1) Establishment of Parkinson's syndrome mouse model
[0045] The Alpha-synuclein (A53T) transgenic mice with the number 004479 were purchased from Jackson Lab in the United States for breeding. Mouse prion promoter. At eight months, some of the homozygous mice progressively developed severe movement impairments. The phenotype emerges by an average of 14-15 months. Skin laxity, weight loss and bradykinesia preceded by dyskinesia, partial paralysis, tremor, and inability to stand. Immunohistochemical analysis of 8- to 12-month-old mutant mice revealed aggregated synuclein inclusions in the spinal cord, brainstem, cerebellum, and striatum. Malignant aggregation of synuclein goes hand in hand with development of movement disorders We used 12-month-old mice as experimental model mice.
[0046] (2) Intravenous injection of TAT-pSyn
[0047] 12-month-old Alpha-synuclein (A53T) model mic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com