Phosphoryl choline coating compositions
a technology of phosphoryl choline and composition, applied in the direction of drug composition, prosthesis, extracellular fluid disorder, etc., can solve the problem that diverse compositions have been used with limited success
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
P(MPC-PEGA-BMA) Copolymer
[0054] The components, 2-methacryloyloxyethyl phosphorylcholine (MPC) butylmethacrylate (BMA), poly(ethylene glycol)acrylate (PEGA) (Mn=350 Da) and AIBN (α,α′-azobutyronitrile) were dissolved in ethanol at a molar ratio of (15:10:74:1). The reactants were maintained at 62° C. for 24 h. The polymer was purified, by a double precipitation in methanol, to yield a white powder.
[0055] A first composition was prepared by mixing the following components: [0056] (a) about 2 mass % poly(butyl methacrylate) (PBMA); [0057] (b) dissolved in a mixture of acetone and cyclohexanone (30% and 70% respectively).
[0058] The first composition was applied onto the surface of a bare 12 mm VISION stent (available from Guidant Corporation) by spraying and dried to form a stent coating. A spray coater was used, having a 0.014 fan nozzle maintained at ambient temperature with a feed pressure of about 0.2 atm (about 3 psi) and an atomization pressure of about 1.3 atm (about 20 psi)....
example 2
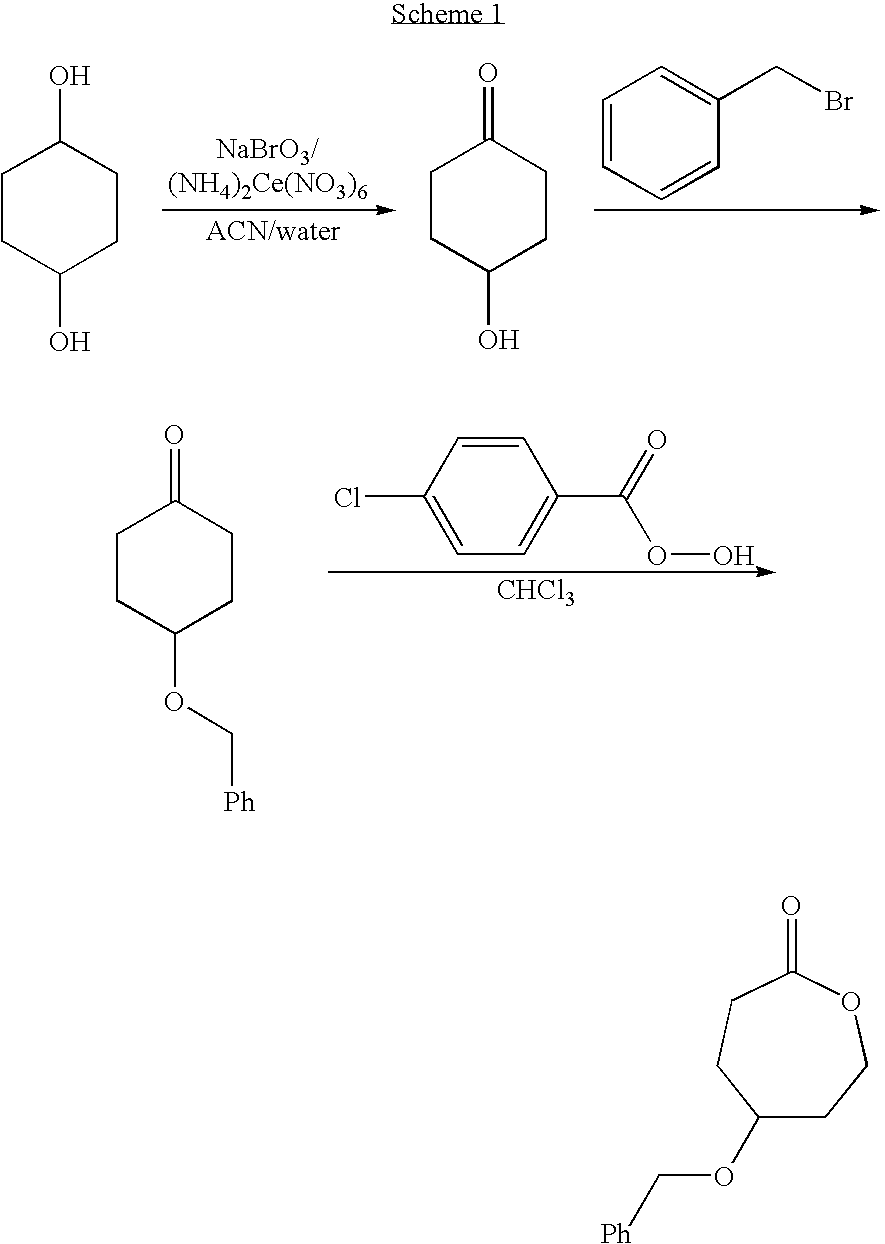
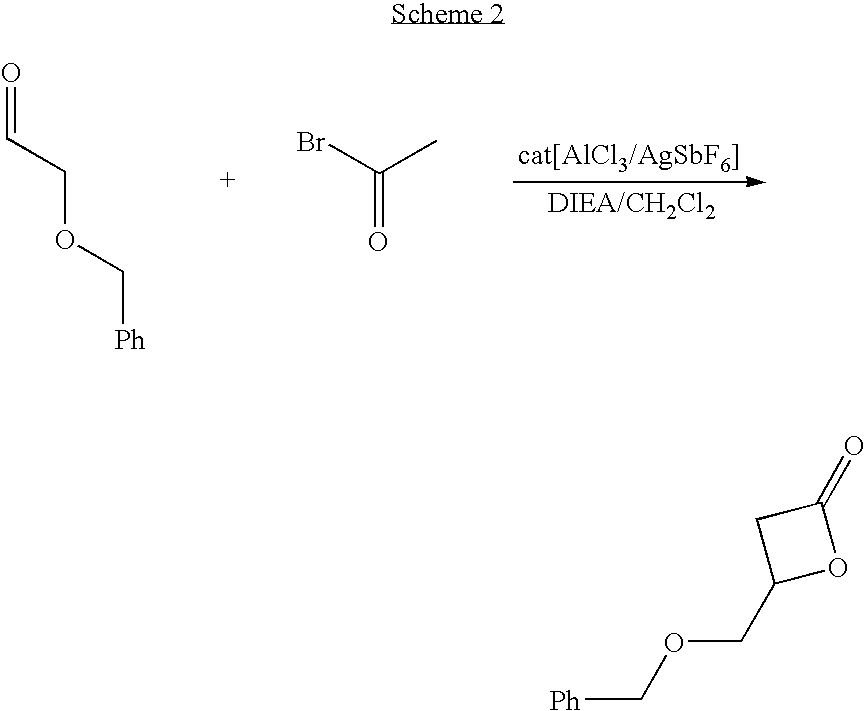
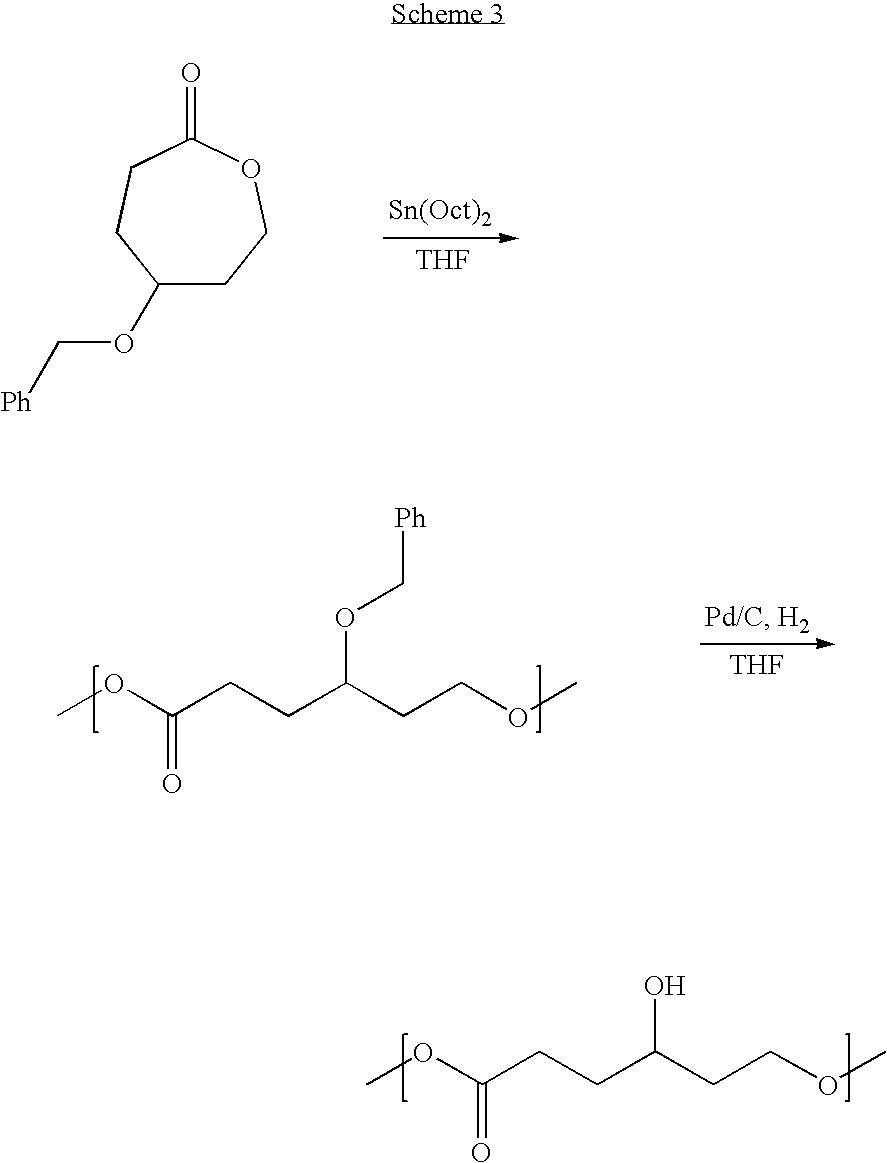
Hydroxyl Functional Caprolactone
[0069] A 100 g 1,4-hexanediol was dissolved in 1.4 L of a mixture of acetonitrile and water (7:3 by volume). A mixture of 45.4 g of sodium bromate and 16.5 g of ammonium cerium (IV) nitrate was slowly added. The reaction was maintained under reflux conditions for 90 min. Once acetonitrile was removed by rotary evaporation, the solution was diluted with 800 mL of water and continuously extracted with chloroform for 72 h. The organic solution was dried over magnesium sulfate. Finally chloroform was evaporated from the organic solution to yield 99.5 g of a colorless oil (4-hydroxycyclohexanone).
[0070] 130 g of benzyl chloride were slowly added to a solution of 60 g of 4-hydroxycyclohexanone in 400 mL of triethylamine. The solution was left to react at 25 ° C. for 2 h. After removal of the solvent, the product was purified by column chromatography to yield 100 g of a white powder 4-benzylestercyclohexanone.
[0071] To a solution of 20 g 3-chloroperoxyben...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Biocompatibility | aaaaa | aaaaa |
| Bioactive | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com