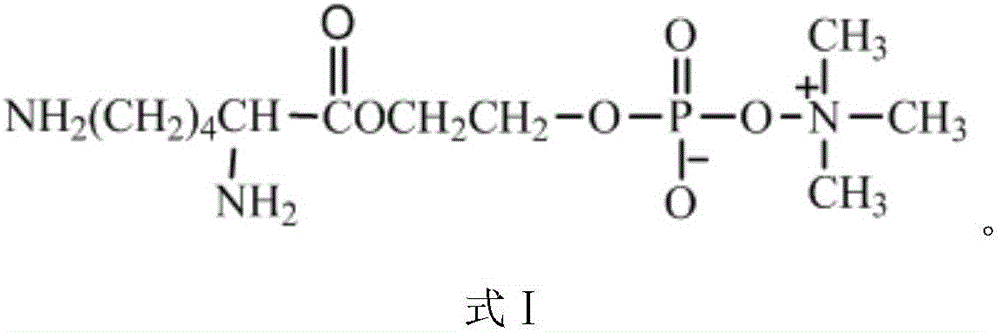Novel bis-amidogen phosphoryl choline compound Lys-EG-PC and preparation method thereof
A phosphorylcholine compound technology, applied in the field of novel diamino phosphorylcholine compound Lys-EG-PC and its preparation, can solve the problems of low reactivity, non-stable existence for a long time, low reaction rate, etc. Achieve the effects of improving activity and stability, improving biocompatibility, and inhibiting protein adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Step 1. Dissolve 0.132mol of Cbz-Cl in 100mL of diethyl ether solution, add dropwise 200mL of 10% NaCO containing 0.055molL-lysine 3 After the dropwise addition, keep the temperature at 0°C for 5 hours. The obtained product is acidified with an appropriate amount of 10% citric acid and then extracted with chloroform. After the product is washed with water, it is evaporated to dryness to obtain a large amount of N,N'-bisbenzyloxycarbonyl-L- Lysine, about 93% yield.
[0039] Step 2. Add 0.055mol of N,N'-bisbenzyloxycarbonyl-L-lysine into a dry 500mL three-necked flask, add 0.11mol of triethylamine acid-binding agent and 200mL of anhydrous tetrahydrofuran, mix well, and put Dissolve 0.145 mol of thionyl chloride in 50 mL of acetone, put it into a constant pressure dropping funnel, and set a mechanical stirrer at -15°C to control the rate of addition of thionyl chloride. After about 2 hours, the addition of thionyl chloride is completed. After about 2 hours after the reacti...
specific example 2
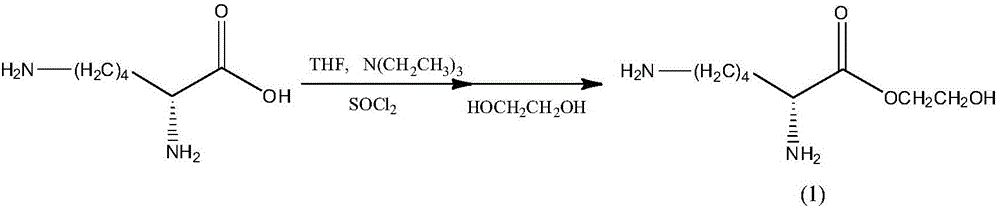
[0044] Step 1. Add 0.055mol of L-lysine and 0.11mol of triethylamine into a 500ml dry three-neck flask, preferably dissolve in 200mL of tetrahydrofuran, keep the reaction temperature at -15°C, and slowly add the solution dropwise under mechanical stirring. Add 0.145 mol thionyl chloride in acetone, drop it within 2 hours, keep the temperature and continue the reaction for 2 hours; after raising the temperature to room temperature, slowly add 0.08 mol ethylene glycol dropwise, react for 6 hours, pour the reaction solution out and suction filter A light yellow solution was obtained, diethyl ether was used three times, and the solvent was evaporated to obtain the product with a yield of about 96%.
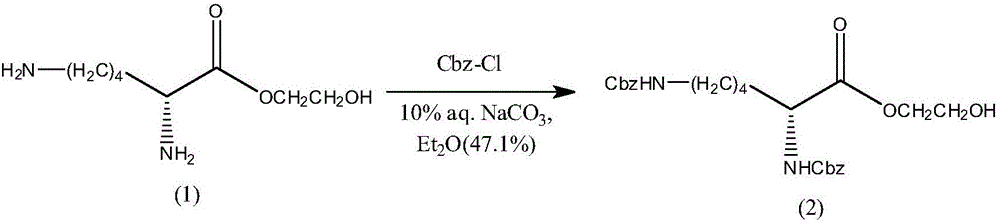
[0045] In step 2, 0.155 mol of Cbz-Cl was dissolved in 100 mL of diethyl ether solution, and 200 mL of 10% NaCO containing 0.0645 mol of compound (2) was added dropwise. 3 Aqueous solution, keeping the temperature at 0°C, reacting for 5 hours, the obtained product was acidified with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com