Peptide analogues of GIP for treatment of diabetes, insulin resistance and obesity
a peptide and insulin resistance technology, applied in the field of insulin release and blood glucose concentration control, can solve the problems of affecting the therapeutic role of incretin hormones, impairing oral glucose tolerance and glycemic response to nutrient ingestion, and debilitating diabetic complications and premature death, so as to enhance the capacity to stimulate insulin secretion, delay glucose absorption, and enhance glucose disposal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N-Terminally Modified GIP and Analogues Thereof
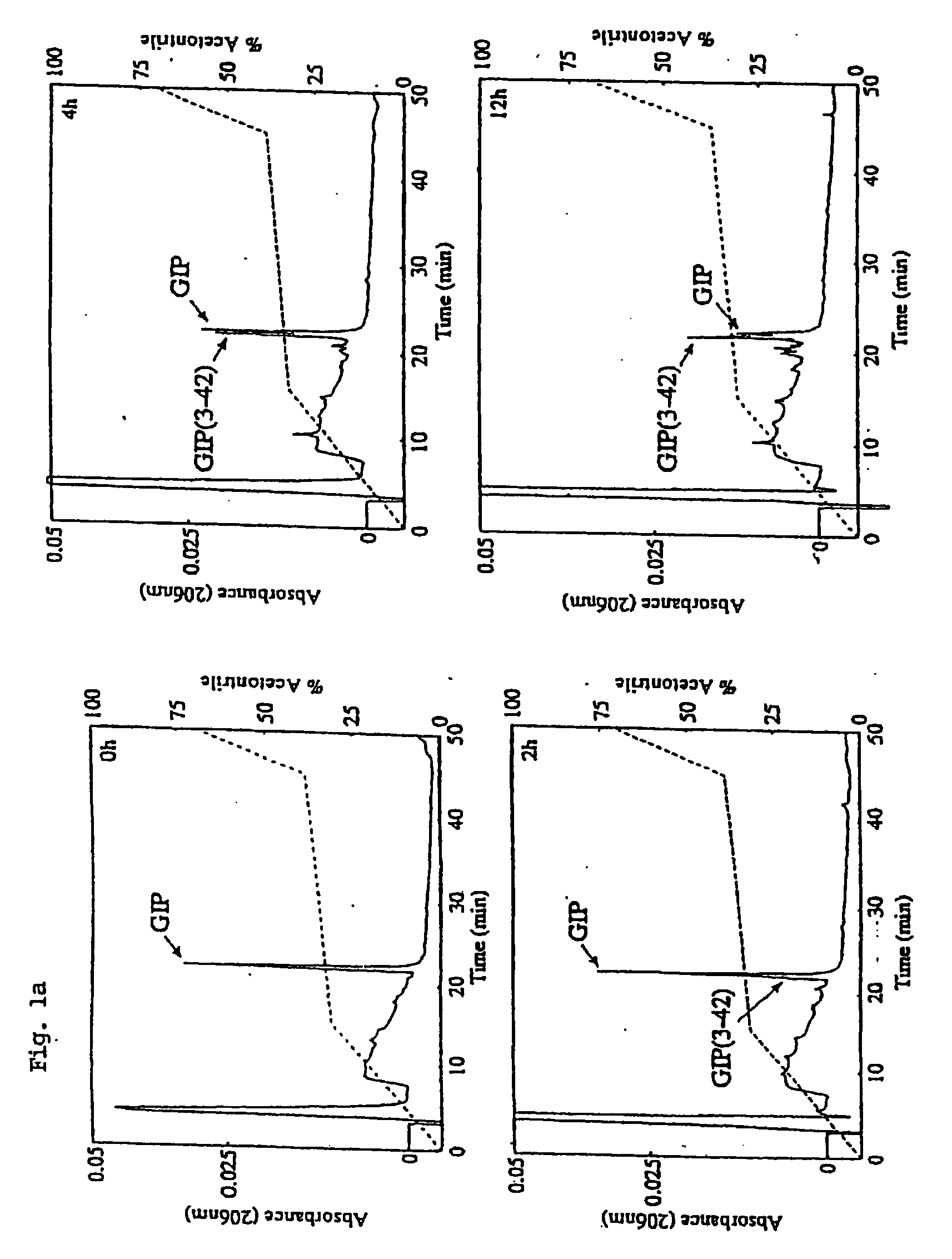
[0173] The N-terminal modification of GIP is essentially a three step process. Firstly, GIP is synthesized from its C-terminal (starting from a Fmoc-Gln (Trt)-Wang resin (Calbiochem Novabiochem, Beeston, Nottingham, UK) up to the penultimate N-terminal amino-acid (Ala2) on an automated peptide synthesizer (Applied Biosystems, California, USA). The synthesis-follows standard Fmoc peptide chemistry protocols. Secondly, the N-terminal amino acid of native GIP (Tyr) is added to a manual bubbler system as a Fmoc-protected Tyr(tBu)-Wang resin. This amino acid is deprotected at its N-terminus (piperidine in DMF (20% v / v)) and allowed to react with a high concentration of glucose (glycation, under reducing conditions with sodium cyanoborohydride), acetic anhydride (acetylation), pyroglutamic acid (pyroglutamyl) etc. for up to 24 hours as necessary to allow the reaction to go to completion. The completeness of reaction is monitor...
example 2
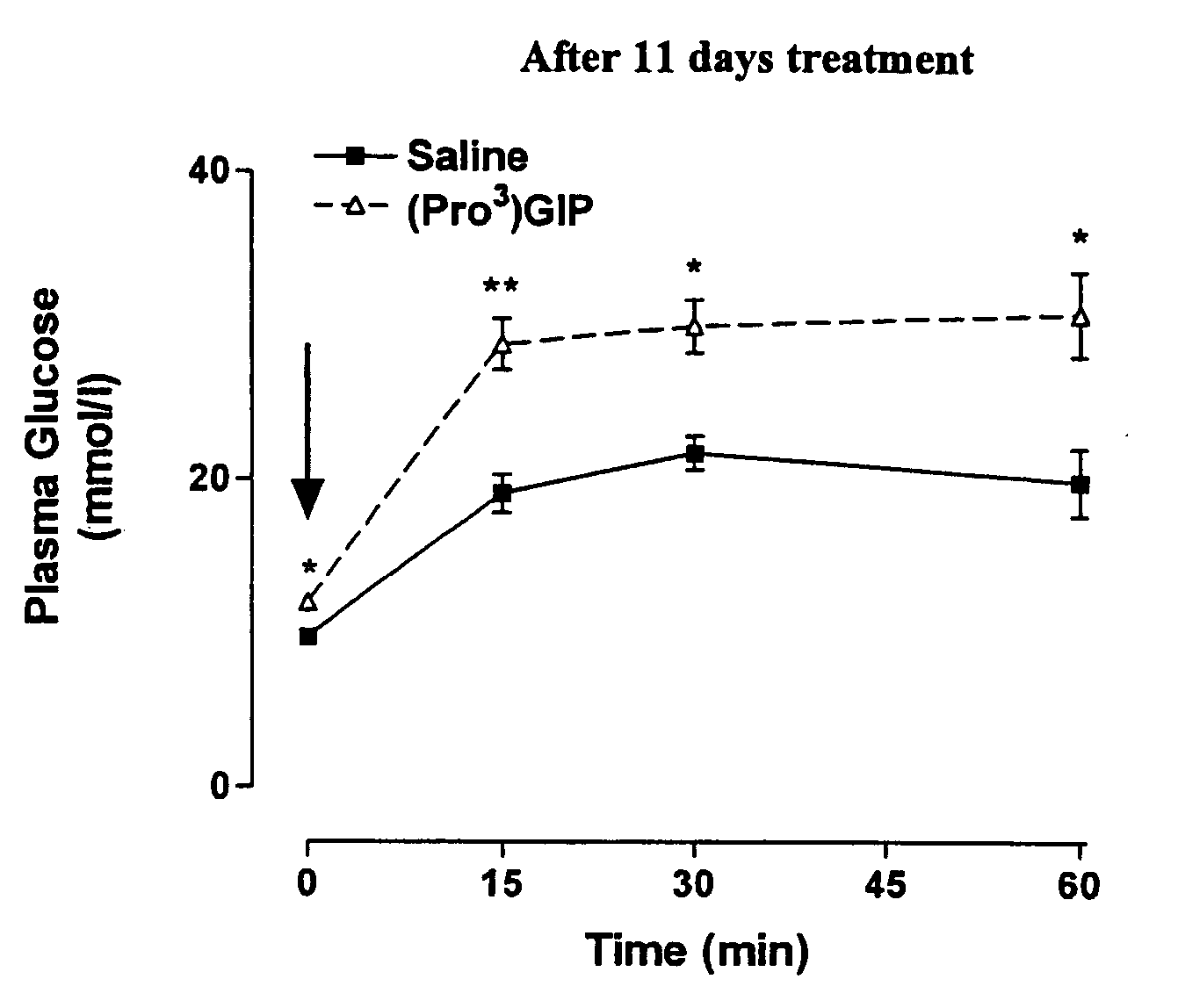
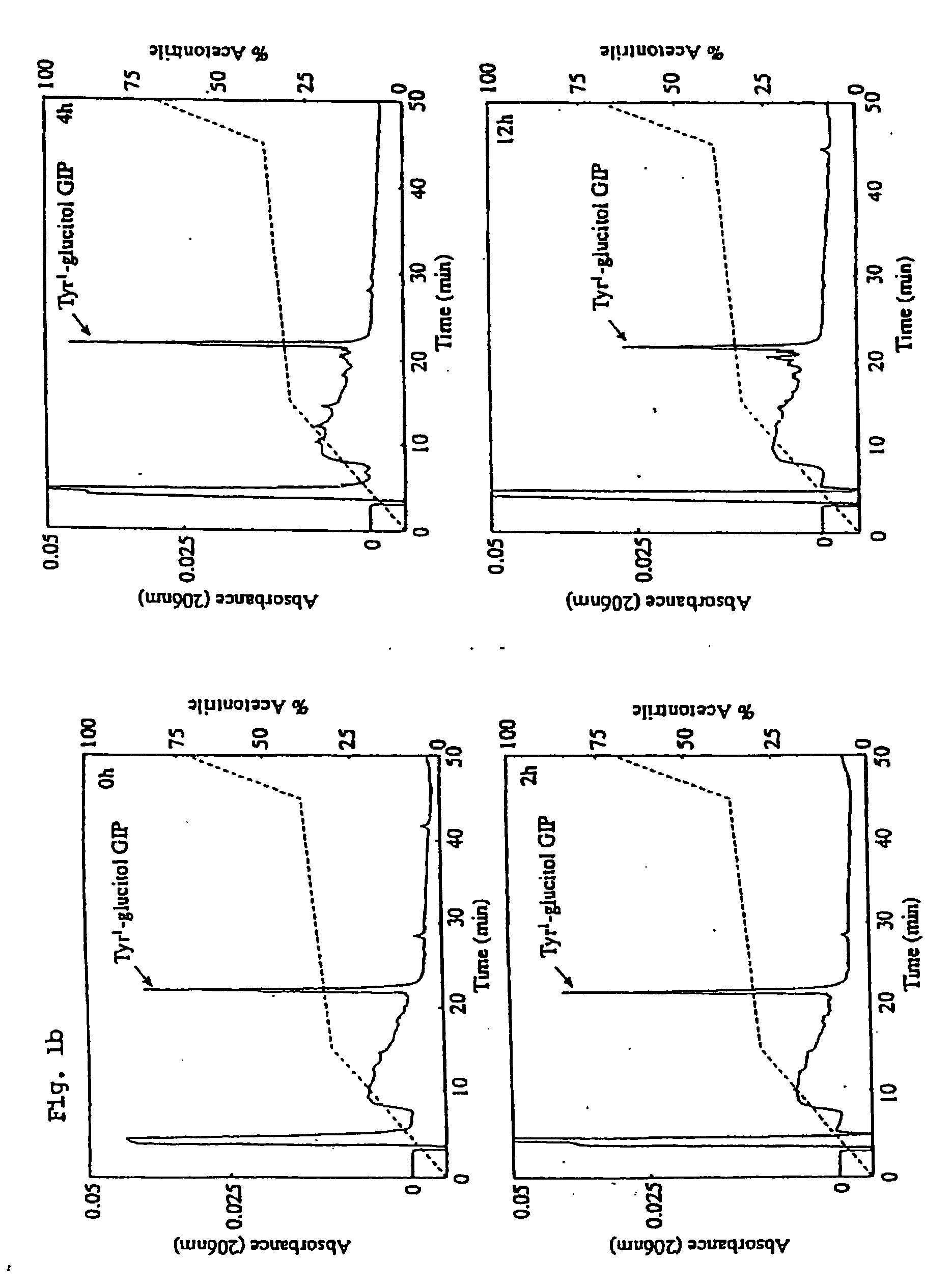
Preparation of Tyr1-Glucitol GIP and its Properties In Vivo
[0174] The following example investigates preparation of Tyr1 glucitol GIP together with evaluation of its antihyperglycemic and insulin-releasing properties in vivo. The results clearly demonstrate that this novel GIP analogue exhibits a substantial resistance to aminopeptidase degradation and increased glucose lowering activity compared with the native GIP.
Research Design and Methods
[0175] Materials. Human GIP was purchased from the American Peptide Company (Sunnyvale, Calif., USA). HPLC grade acetonitrile was obtained from Rathburn (Walkersburn, Scotland). Sequencing grade trifluoroacetic acid (TFA) was obtained from Aldrich (Poole, Dorset, UK). All other chemicals purchased including dextran T-70, activated charcoal, sodium cyanoborohydride and bovine serum albumin fraction V were from Sigma (Poole, Dorset, UK). Diprotin A (DPA) was purchased from Calbiochem-Novabiochem (UK) Ltd. (Beeston, Nottingham, UK) and rat ins...
example 3
Additional N-Terminal Structural Modifications of GIP
[0190] This example further looked at the ability of additional N-terminal structural modifications of GIP in preventing inactivation by DPP and in plasma and their associated increase in both the insulin-releasing potency and potential therapeutic value. Native human GIP, glycated GIP, acetylated GIP and a number of GIP analogues with N-terminal amino acid substitutions were tested.
[0191] Materials and Methods. High-performance liquid chromatography (HPLC) grade acetonitrile was obtained from Rathburn (Walkersburn, Scotland). Sequencing grade trifluoroacetic acid (TFA) was obtained from Aldrich (Poole, Dorset, UK). Dipeptidyl peptidase IV was purchased from Sigma (Poole, Dorset, UK), and Diprotin A was purchased from Calbiochem Novabiochem (Beeston, Nottingham, UK). RPMI 1640 tissue culture medium, foetal calf serum, penicillin and streptomycin were all purchased from Gibco (Paisley, Strathclyde, UK). All water used in these ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameters | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com