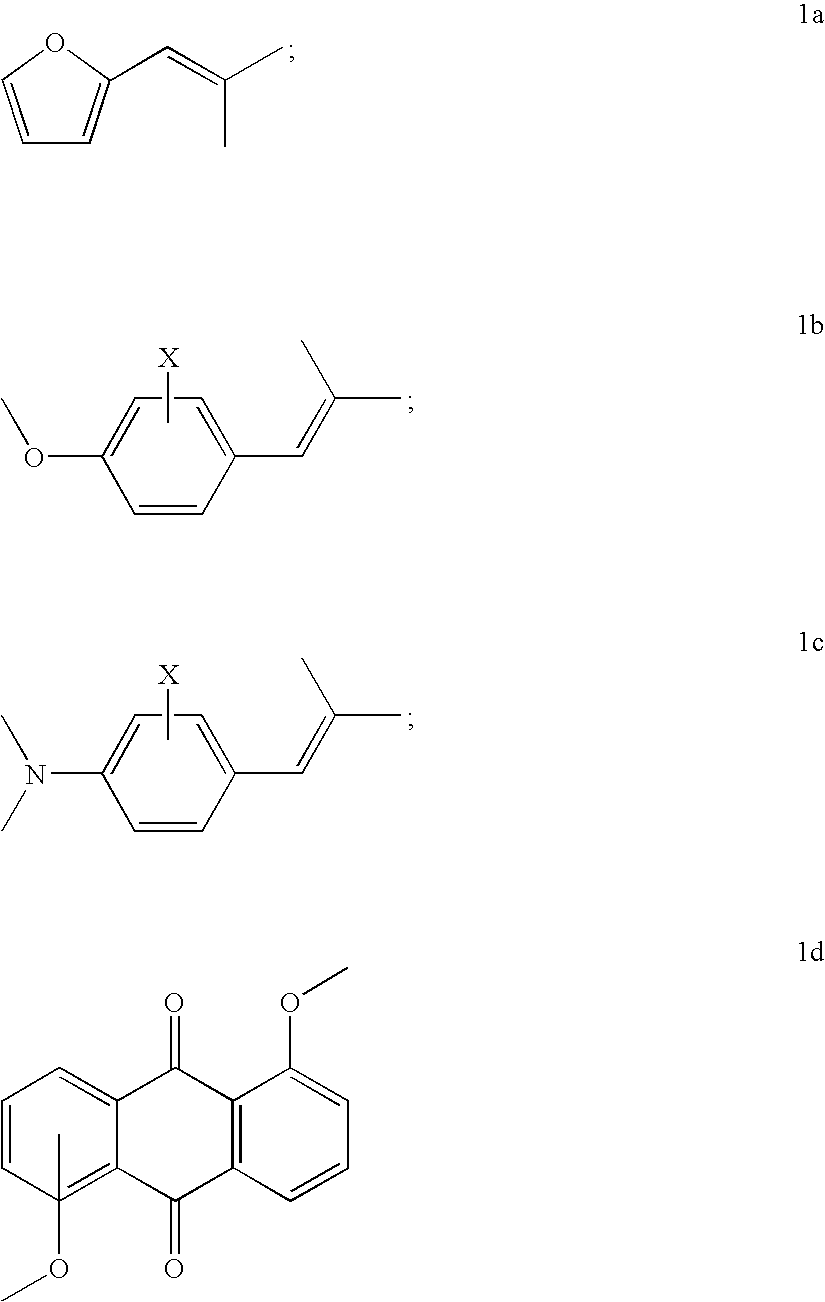
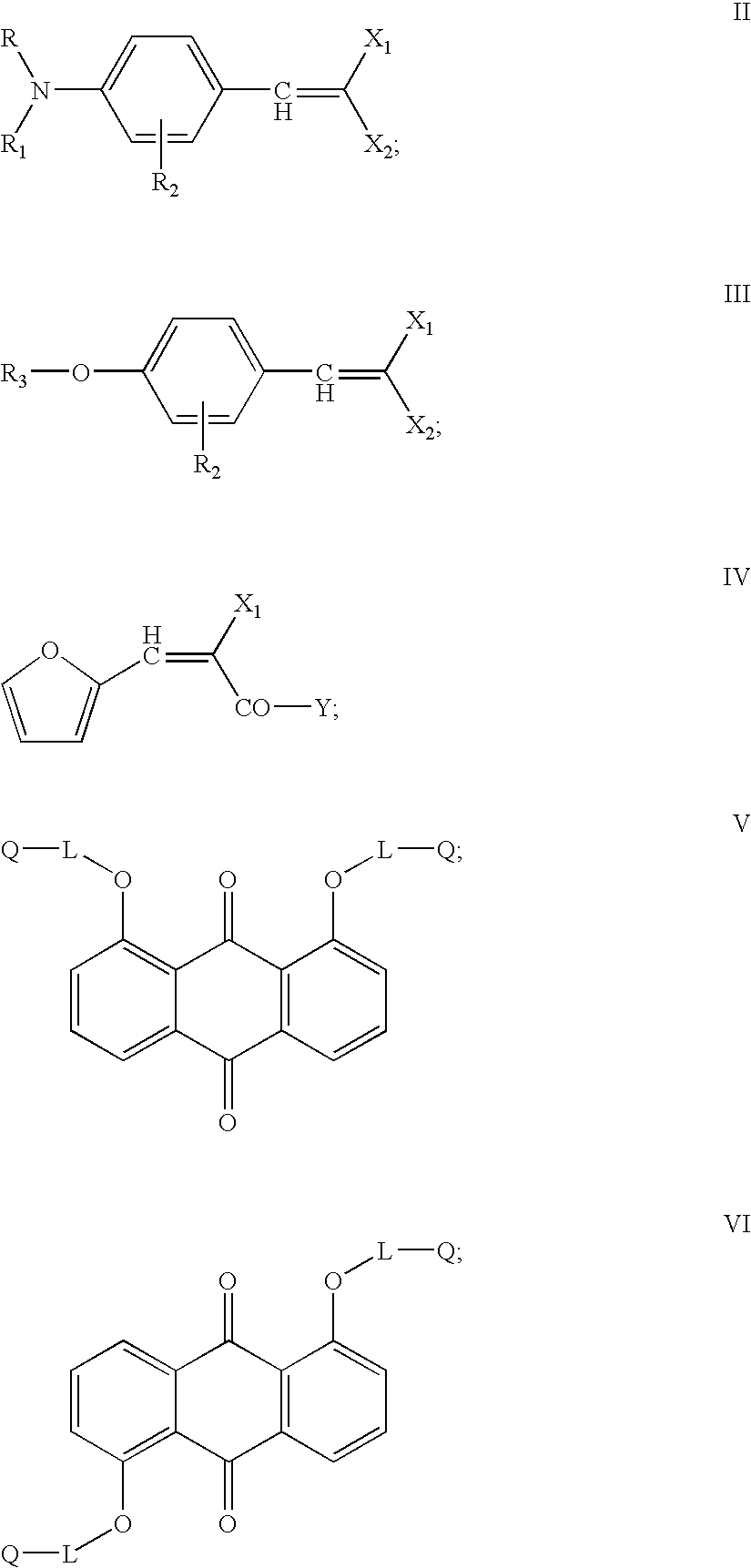
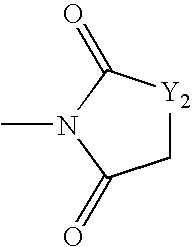
Copolymerizable methine and anthraquinone compounds and articles containing them
a technology of polymerizable methine and anthraquinone, which is applied in the field of polymerizable ultraviolet light absorbers, yellow colorants, can solve the problems of increasing production and materials costs, unsuitable for use in artificial lens materials, and complicated manufacturing processes, so as to reduce the potential for compound leaching out of materials, reduce the intensity of violet-blue light transmitted, and the effect of reducing the absorption properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 104
[0127]
[0128] To a clean, dry 1 L 4-neck flask equipped with a mechanical stirrer, heating mantle, thermocouple, addition funnel and a Dean-Stark trap were added 99 g of methyl cyanoacetate (1.0 mol). The reaction vessel was stirred under an atmosphere of dry nitrogen and heated to 95° C. To the reaction vessel were added 61.1 g (1.0 mol) of ethanolamine at a dropwise rate while removing low boilers via the Dean-Stark trap. An exotherm occurred early during the addition and the temperature increased to 105° C. The addition was complete in about 45 minutes and the reaction vessel temperature was increased to 150° C. After about 1 h low boilers were no longer being collected in the Dean-Stark trap. The reaction solution was allowed to cool to about 85° C. and 125 mL of cyclohexane was added at a rapid, dropwise rate. The cyclohexane was decanted. A fresh 75 mL of cyclohexane were added and the warm mixture was transferred to a 600 mL beaker. The product began to solidify to give a waxy...
example 105
[0129]
[0130] To a clean, dry 500 mL flask equipped with a heating mantle and addition funnel were added 200 mL of DMF and 50.7 g of N-phenylthiomorpholine-S,S-dioxide (0.2 mol, available from Eastman Chemical Company). The reaction vessel was purged with nitrogen and 20.5 mL of phosphorus oxychloride (0.2 mol) were added at such a rate to keep the temperature from exceeding 35° C. The reaction vessel was heated to 80-90° C. and stirred for about 4 h. The reaction mixture was allowed to cool to room temperature and poured into a mechanically stirred ice water mixture (1 L) containing 100 mL of concentrated ammonium hydroxide. A white precipitate formed that was collected by suction filtration and washed with water. The cake was allowed to dry on the filter overnight to give 54.4 g of product.
example 106
[0131]
[0132] To a 500 mL round-bottomed flask equipped with a mechanical stirrer, reflux condenser and heating mantle were added methanol (175 mL), 18.0 g of product from Example 105 (75.0 mmols) and 10.4 g of product from Example 104 (80.0 mmols). The mixture was heated to reflux and stirred for 3 h then allowed to cool to room temperature, at which time a precipitate formed. The precipitate was collected by suction filtration, washed with cold methanol and allowed to dry on the filter overnight to give 19.75 g of light yellow compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelengths | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com