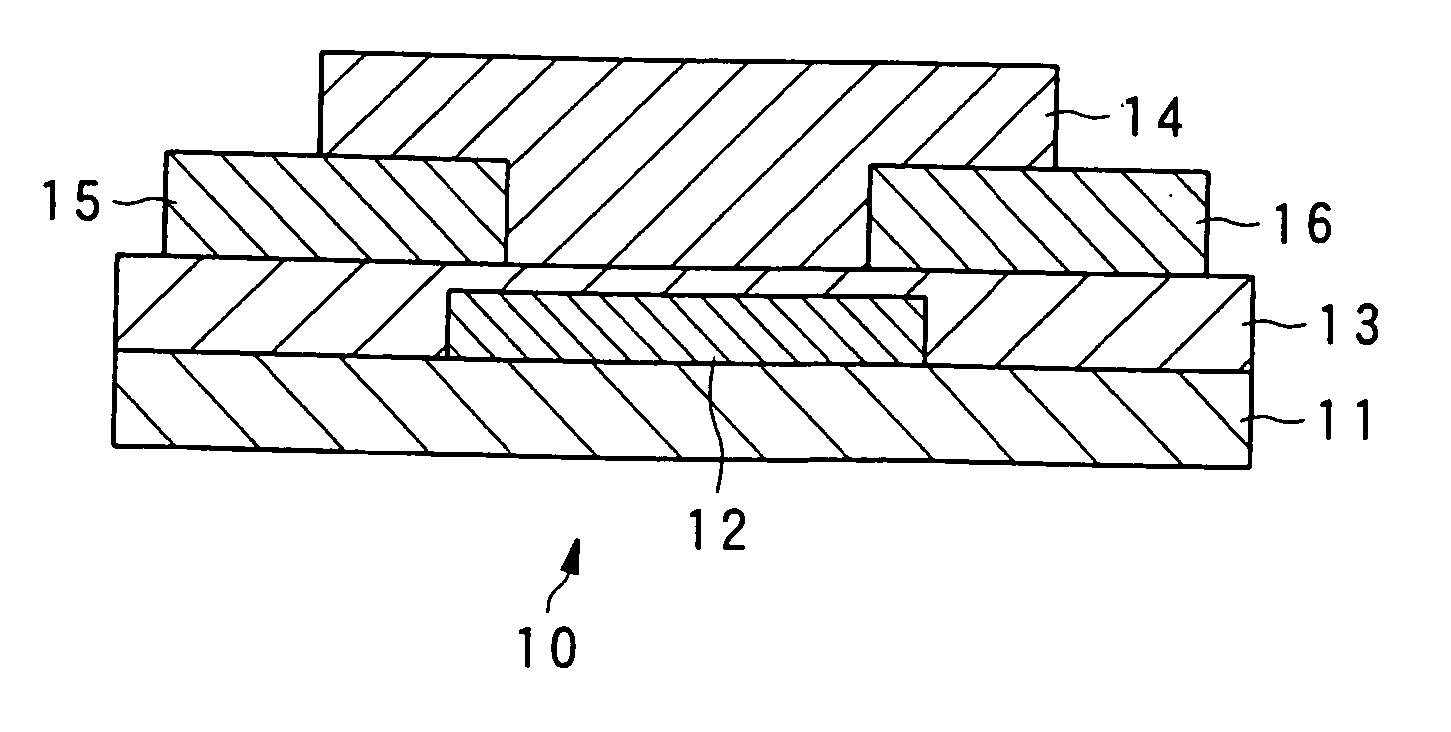
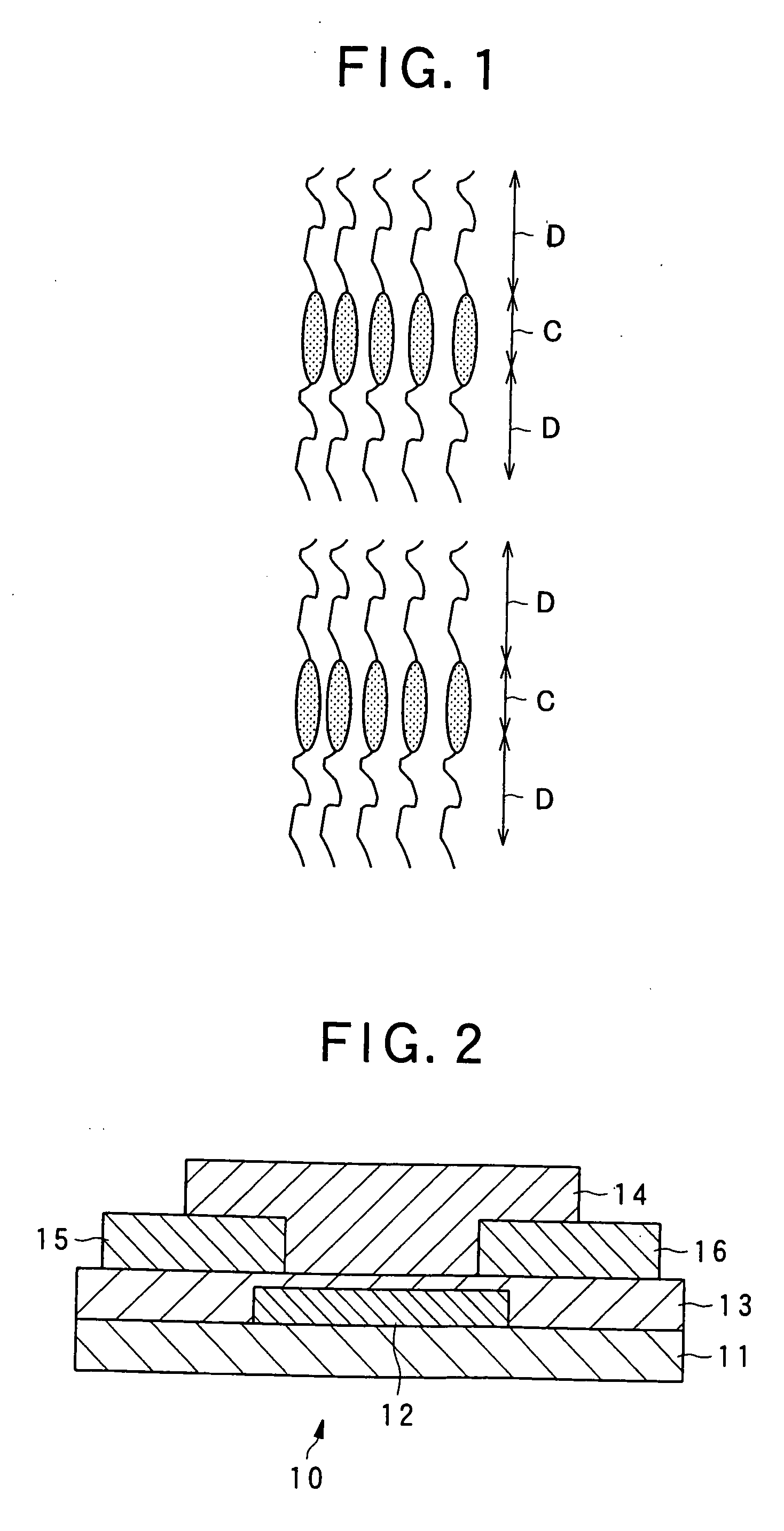
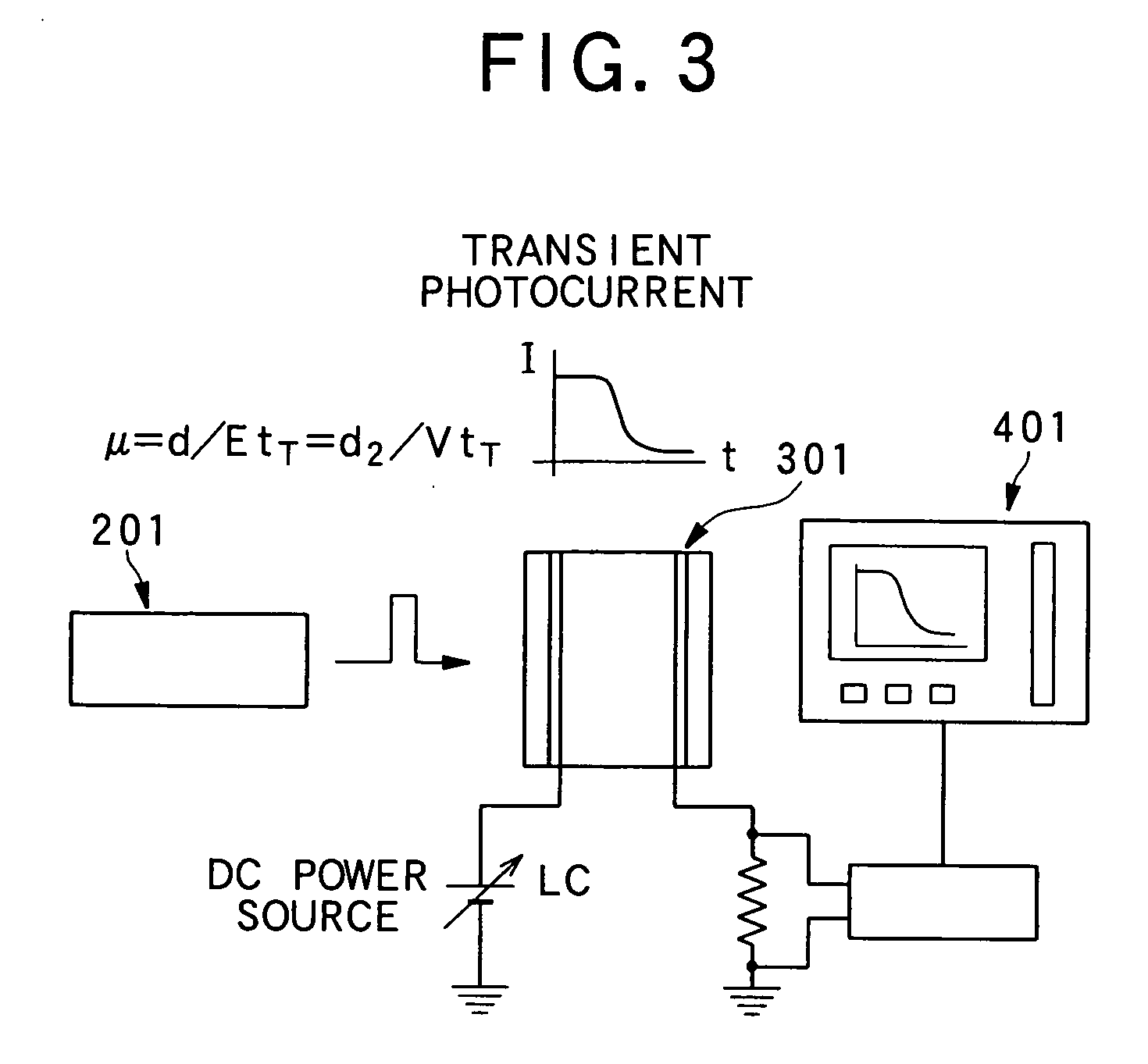
Liquid crystalline organic compound, organic semiconductor structure, organic semiconductor device, and process for producing liquid crystalline organic compound
a technology of organic compound and liquid crystal, which is applied in the direction of organic chemistry, chemistry apparatus and processes, and organic chemistry. it can solve the problems of large number of grain boundaries that are liable to be present, defects that are easy to be generated, and defects that hinder the transportation of charges. achieve the effect of high charge mobility and effective production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 2-(4′-heptyloxyphenyl)-6-dodecylbenzothiazole (abbreviated to 70-PBT-12 hereinafter)
[0065]
[0066] Acetic acid (900 ml) was added to 4-dodecylaniline (60 g, 229.4 mmol) and potassium thiocyanate (55.7 g, 573.8 mmol), and the resultant solution was stirred at 25° C. for 1 hour. While the solution was cooled in an ice bath, a solution of bromine in acetic acid (Br2 / AcOH=14.1 ml / 150 ml) was dropwise added thereto, and the resultant was stirred at 25° C. for 24 hours. After the stirring, hot water (500 ml) was added to the solution, and the resultant was filtrated. Thereafter, ammonium water was added to the filtrate until the solution turned alkaline. The precipitated solid was extracted with ethyl acetate, and further isolated with a column chromatography (developing solvent was hexane:ethyl acetate=1:2). Thereafter, the solid was recrystallized from ethyl acetate to yield 43.8 g of 2-amino-6-dodecylbenzothiazole (yield: 41.8%).
[0067] Next, water (100 ml) was added to 2-a...
example 2
Synthesis of 2-(4′-octylphenyl)-6-butoxybenzothiazole (abbreviated to 8-PBT-04 hereinafter)
[0071]
[0072] Acetic acid (200 ml) was added to 4-butoxyaniline (25 g, 151 mmol) and potassium thiocyanate (36.7 g, 378 mmol), and the resultant solution was stirred at 25° C. for 1 hour. While the solution was cooled in an ice bath, a solution of bromine in acetic acid (Br2 / AcOH=9.3 ml / 50 ml) was dropwise added thereto, and the resultant was stirred at 25° C. for 24 hours. After the stirring, hot water (125 ml) was added to the solution, and the resultant was filtrated. Thereafter, ammonium water was added to the filtrate until the solution turned alkaline. The precipitated solid was extracted with ethyl acetate, and further isolated with a column chromatography (developing solvent was hexane: ethyl acetate=1:2). Thereafter, the solid was recrystallized from ethyl acetate to yield 17.1 g of 2-amino-6-butoxybenzothiazole (yield: 51%).
[0073] Next, water (50 ml) was added to 2-amino-6-butoxyben...
example 3
Synthesis of 2-(4′-octylphenyl)-6-dodecylbenzothiazole (abbreviated to 8-PBT-12 hereinafter)
[0077]
[0078] Acetic acid (900 ml) was added to 4-dodecylaniline (60 g, 229.4 mmol) and potassium thiocyanate (55.7 g, 573.8 mmol), and the resultant solution was stirred at 25° C. for 1 hour. While the solution was cooled in an ice bath, a solution of bromine in acetic acid (Br2 / AcOH=14.1 ml / 150 ml) was dropwise added thereto, and the resultant was stirred at 25° C. for 24 hours. After the stirring, hot water (500 ml) was added to the solution, and the resultant was filtrated. Thereafter, ammonium water was added to the filtrate until the solution turned alkaline. The precipitated solid was extracted with ethyl acetate, and further isolated with a column chromatography (developing solvent was hexane:ethyl acetate=1:2). Thereafter, the solid was recrystallized from ethyl acetate to yield 43.8 g of 2-amino-6-dodecylbenzothiazole (yield: 41.8%).
[0079] Next, water (100 ml) was added to 2-amino-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com