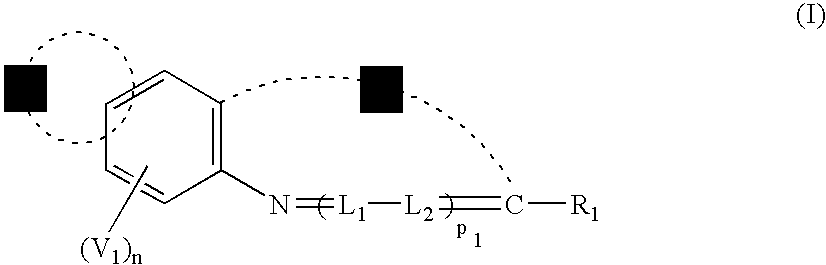
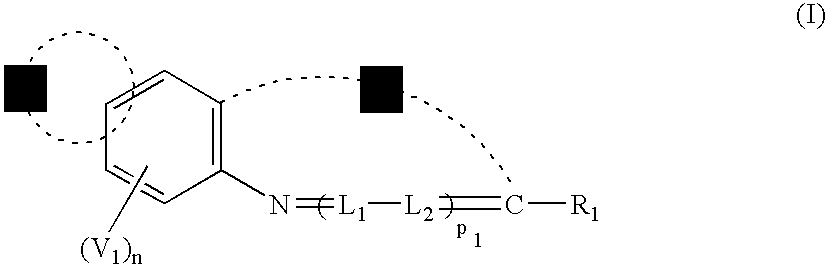
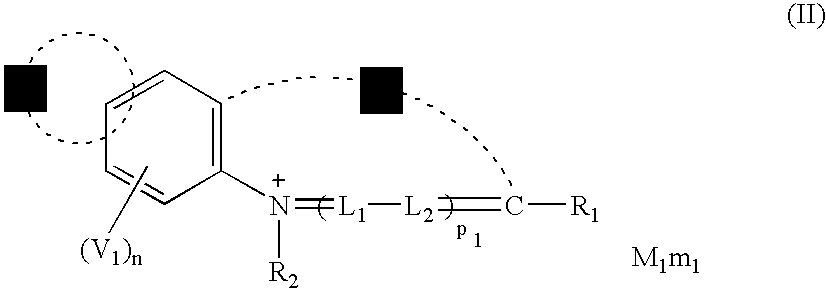
Methine compound and silver halide photographic material containing the same
a technology of silver halide and compound, which is applied in the direction of methine/polymethine dyes, photosensitive materials, instruments, etc., can solve the problems of residual color, insufficient spectral sensitivity of conventional compounds in specific emulsions or specific wavelength regions, and insufficient level of sensitizing dyes contained in silver halide photographic materials, etc., to achieve high-speed silver halide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compound III-41
[0159] Compound III-41 was synthesized according to the following reaction scheme 1.
Synthesis of Compound I-1-e
[0160] 5-Fluoro-2-nitrophenol (10 g) was put into a three-neck flask having a capacity of 300 ml, and 70 ml of DMF was added thereto to dissolve the content. Subsequently, 10 g of potassium carbonate and 10.8 g of benzyl bromide were added to the reaction solution and the solution was stirred for 3 hours at room temperature. Thereto was added 500 ml of water and the reaction mixture was extracted with 500 ml of ethyl acetate. The organic phase was separated, washed with 200 ml of water two times, then with 100 ml of a saturated brine, dried over magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure. Crude crystals of Compound I-1-e thus-obtained was recrystallized with methanol to obtain 7.0 g of Compound I-1-e (yield: 64%).
Synthesis of Compound I-1-d
example 2
Synthesis of Compound III-40
[0169] Compound III-40 was synthesized according to the following reaction scheme.
Synthesis of Compound II-3
[0170] Compound I-1 (0.2 g) and 0.5 g of tolylsultone were mixed and stirred at 150° C. for 3 hours. After the reaction mixture was cooled, 100 ml of ethyl acetate was added thereto, followed by stirring the mixture at room temperature for 2 hours. The crystals were collected by filtration and dried, thereby 0.48 g of Compound II-3 was obtained (yield: 100%, melting point: 279° C.).
Synthesis of Compound III-40
[0171] Compound III-40 (0.48 g), 1.5 ml of triethyl orthopropionate, 1.5 ml of pyridine, 0.6 ml of acetic acid, and 0.5 ml of triethylamine were mixed and stirred at 150° C. for 20 minutes. After the reaction mixture was cooled, 50 ml of ethyl acetate was added thereto, followed by stirring the mixture at room temperature for 30 minutes. The crystals were collected by filtration. The crystals obtained were dissolved in a 1 / 1 mixed solu...
example 3
Synthesis of Compound II-1
[0173] Compound II-1 was synthesized according to the following reaction scheme.
Synthesis of Compound II-1
[0174] Compound I-1 (0.3 g) and 0.5 g of propanesultone were mixed and stirred at 150° C. for 3 hours. After the reaction mixture was cooled, 100 ml of ethyl acetate was added thereto, followed by stirring the mixture at room temperature for 2 hours. The crystals were collected by filtration and dried, thereby 0.44 g of Compound II-1 was obtained (yield: 95%, melting point: 283° C.).
PUM
| Property | Measurement | Unit |
|---|---|---|
| maximum wavelength | aaaaa | aaaaa |
| spectral absorption maximum wavelength | aaaaa | aaaaa |
| spectral absorption maximum wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com