Synthesis of Metallic Nanoparticle Dispersions
a technology of nanoparticles and dispersions, applied in the field of nanoparticles, can solve the problems of limiting the processing of flake-based inks, unable to achieve conductivities of only 2 to 10% of bulk metal conductivity, and the current method of printing conductive compositions onto substrates has certain limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
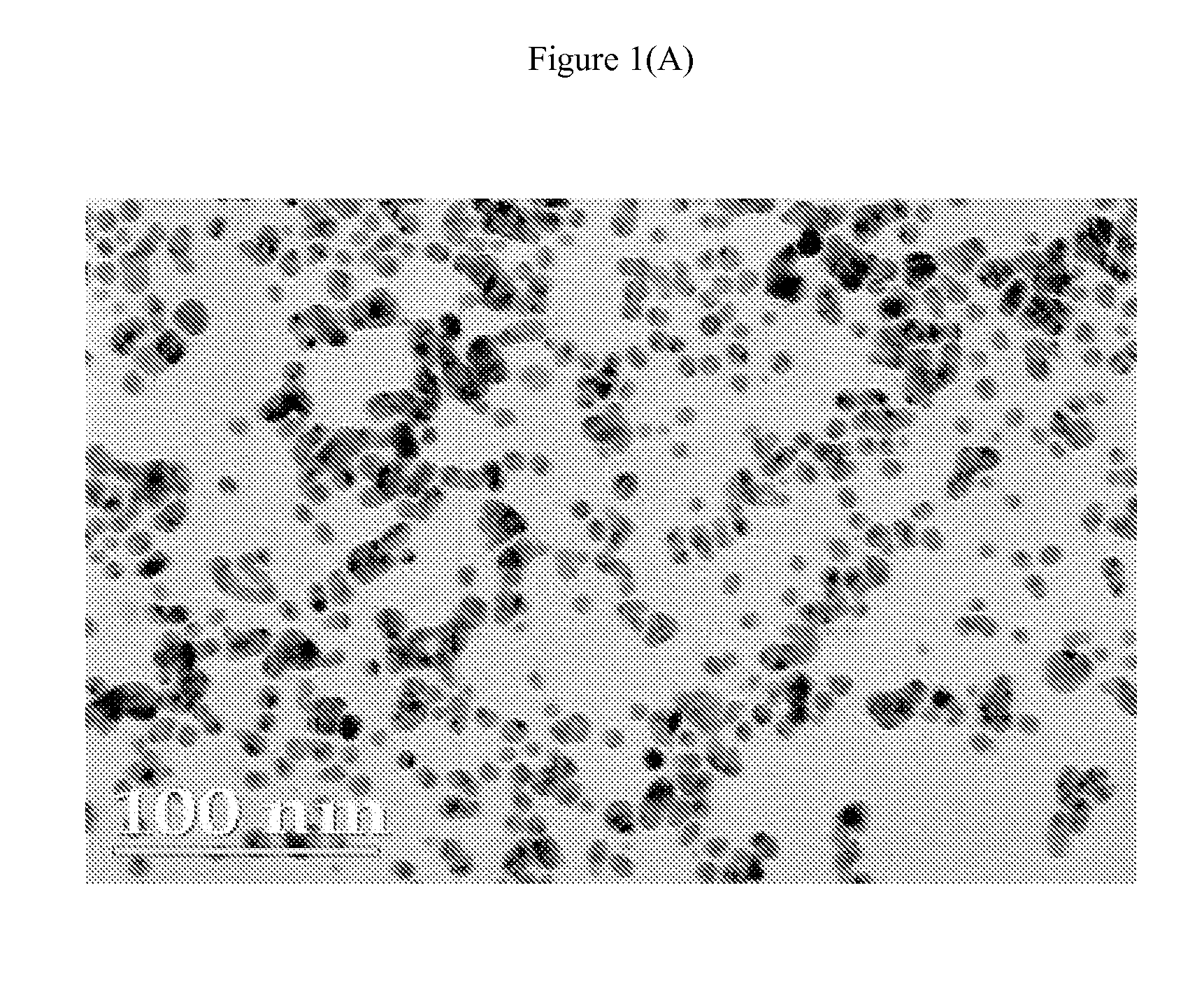
[0092] An initial solution was prepared by adding 7.5 grams of ammonium hydroxide (30% ammonia by weight) to 275 grams of water; 13.5 grams of heptanoic acid was added to this solution followed by 20.9 grams of 50% hydrazine hydrate aqueous solution. The ammonium hydroxide is necessary to allow the acid to dissolve in the water. Separately, 36 grams of silver nitrate was dissolved in 175 grams of water. The silver nitrate solution was added to the initial solution while stirring under nitrogen. The resultant product was flocculated and allowed to settle. Excess water was decanted off. The concentrated product was spread onto 5 mil polyester film with a 0.5 mil wire wound rod and then cured at 80° C. and 100° C. for 1-2 minutes resulting in cohesive and conductive silver films.
example 2
[0093] An initial solution was prepared by adding 2.1 grams of ammonium hydroxide (30% ammonia by weight) to 50 grams of water; 7.8 grams of heptanoic acid was added to this solution followed by 3 grams of 50% hydrazine hydrate aqueous solution. Separately, 10 grams of silver nitrate was dissolved in 50 grams of water. The silver nitrate solution was added to the initial solution while stirring under nitrogen. The resultant product was allowed to settle and the excess water decanted off.
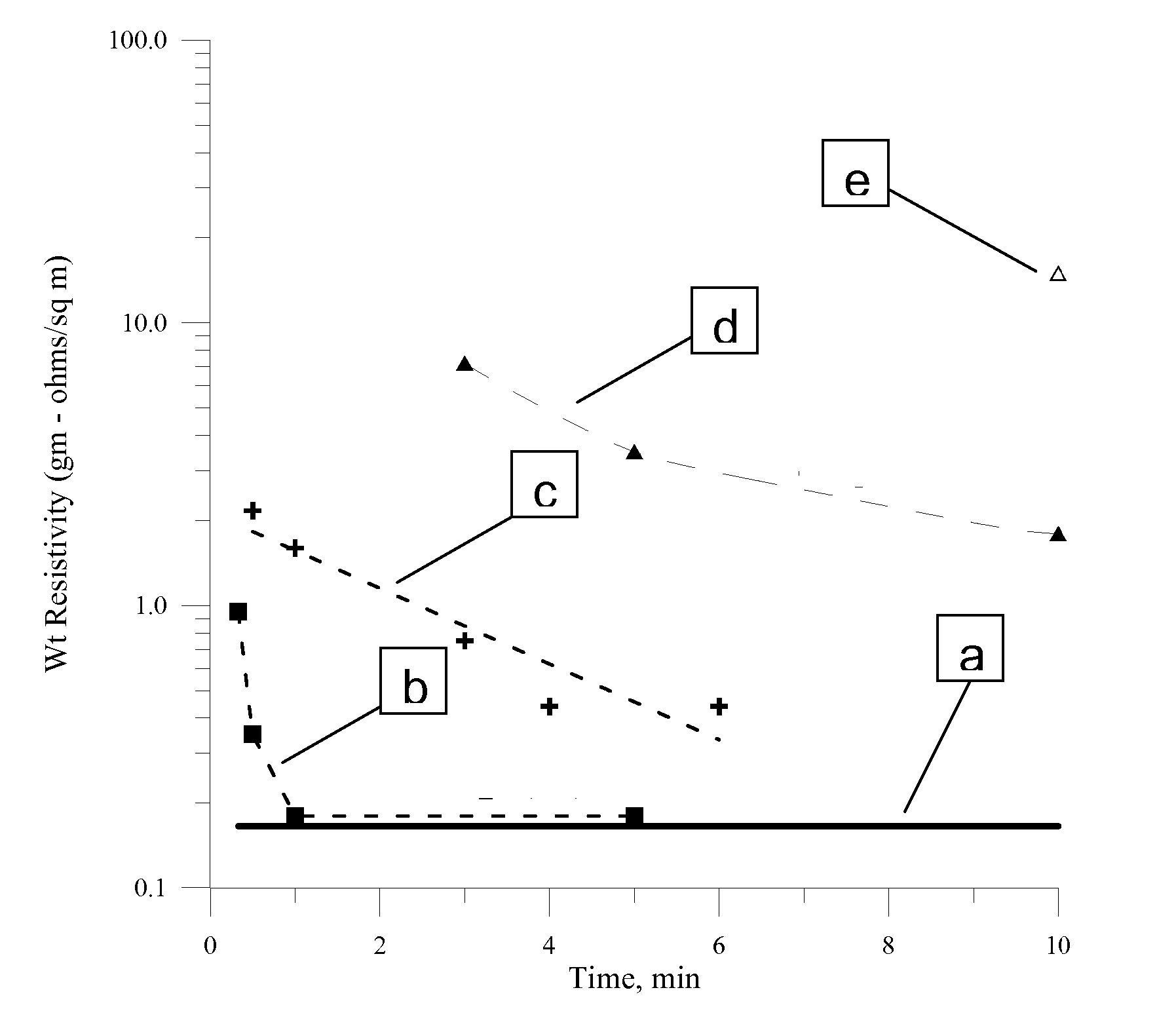
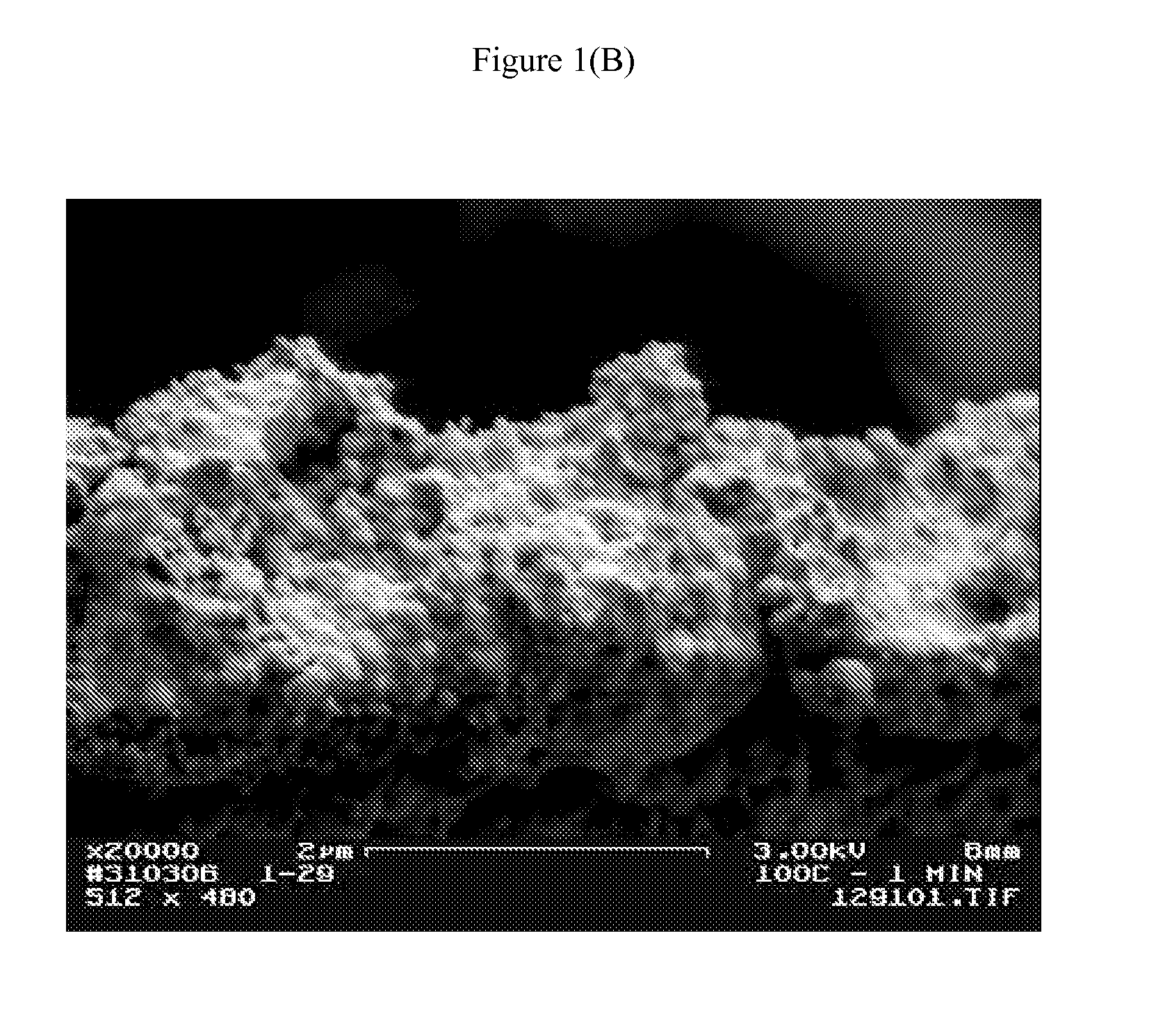
[0094] The concentrated product was spread onto 5 mil polyester film with a 0.5 mil wire wound rod and then cured at 80° C. and 100° C. for 1-2 minutes resulting in cohesive and conductive silver films. The weight resistivity of a sample cured at 100° C. for 1 minute was measured to be 0.39 gram-ohms / m2 (˜2×bulk silver).
example 3
[0095] An ink composition was prepared by adding 50 grams of spherical silver powder (1-2 um mean diameter) to 50 grams of 35 wt % nanoparticle dispersion of Example 1 also containing 3 wt % of an acrylic copolymer latex (55 wt % polymer), 2 wt % of polyvinyl alcohol (25 wt % in water, Mw of 8,000-9,000), and 1 wt % ethylene glycol. The materials were mixed well together, and were milled in a mortar and pestle until a homogeneous mixture was obtained. A film of the resulting ink was deposited onto 0.005″ thick untreated polyester film with a 0.0015″ Bird film applicator. The wet film was cured in a 100° C. for 30 seconds followed by 60 seconds at 140° C. The weight resistivity of the resulting silver films was measured to be 1.3 gram-ohms / m2, approximately 8 times the resistivity of bulk silver. The adhesion of the film to the substrate was tested by applying a 4″ long strip of Scotch brand tape (3M Corporation) to the film, insuring good adhesion to the film by applying pressure wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com