Methods and assays useful in the treatment of alcohol dependence or alcohol abuse
a technology of alcohol dependence and assays, applied in the direction of biocide, drug composition, peptide/protein ingredients, etc., can solve the problems of high cost of alcohol dependence and abuse, alcohol abuse and dependence, etc., to reduce alcohol consumption, increase the concentration of aldehydes, and alter the concentration of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Compounds
[0045] Daidzin and structurally related compounds were either commercially available or prepared according to standard techniques. See U.S. Pat. No. 5,624,910 previously incorporated by reference in its entirety. Daidzin analogs within the scope of the present invention not only include the compounds represented by formula I but also those wherein the glucose is replaced with a different sugar moiety. For example, L and D aldo- or keto-tetroses, pentoses, hexoses, heptoses or the amino, alcohol and / or acid derivatives of such tetroses, pentoses, hexoses or heptoses; or wherein the glucose is replaced by the deoxy analogs of such tetroses, pentoses, hexoses or heptoses. Alternatively, the glucose (GlcO) moiety of daidzin may be replaced by alkoxy or acyloxy groups at the 7-position bearing various chain lengths, for example, up to 11 or more, comprising any of straight chain alkyl, peptidic, polyether, etc. backbones, and the backbones may be substituted with various neutra...
example ii
ALDH-Inhibitory Activity
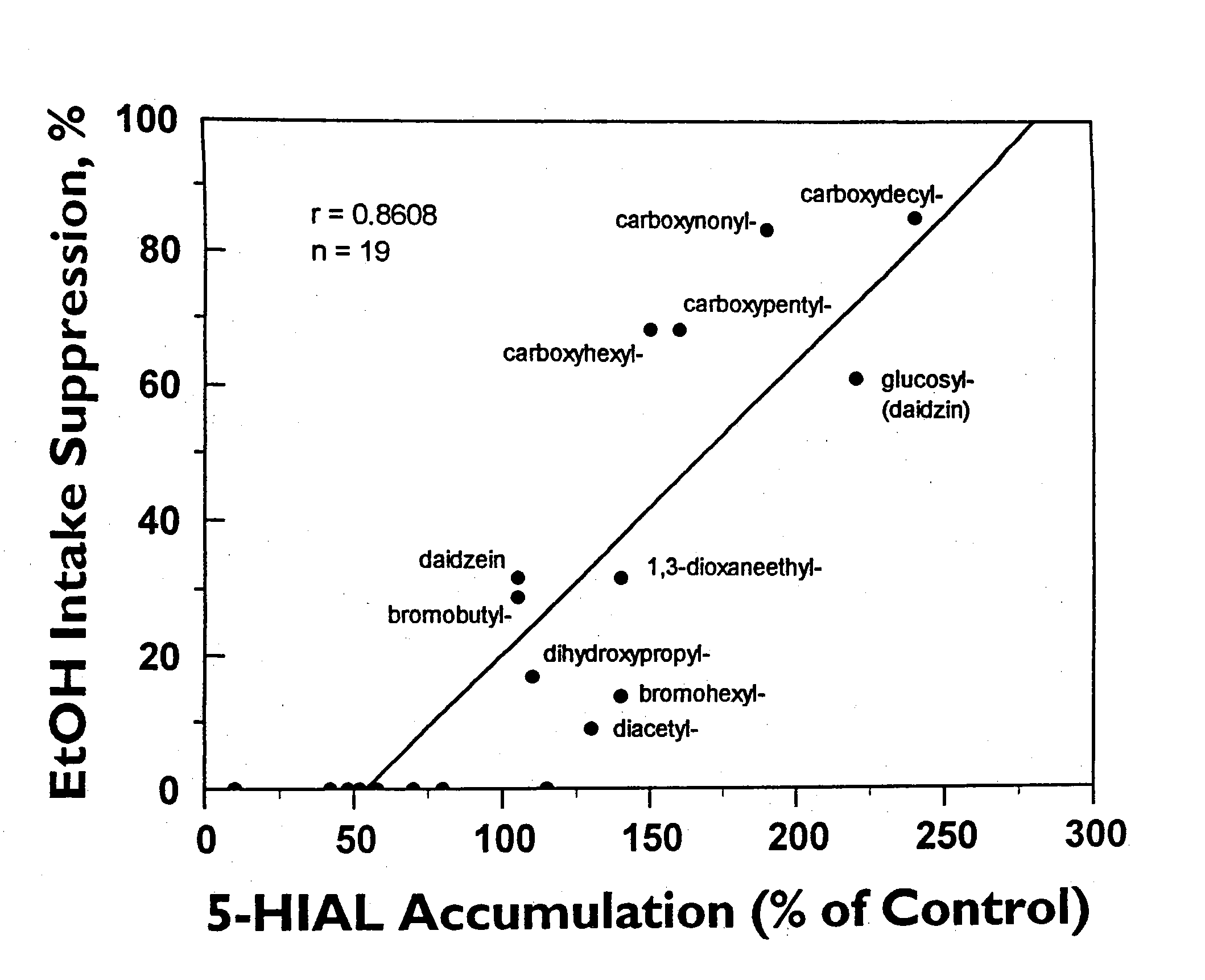
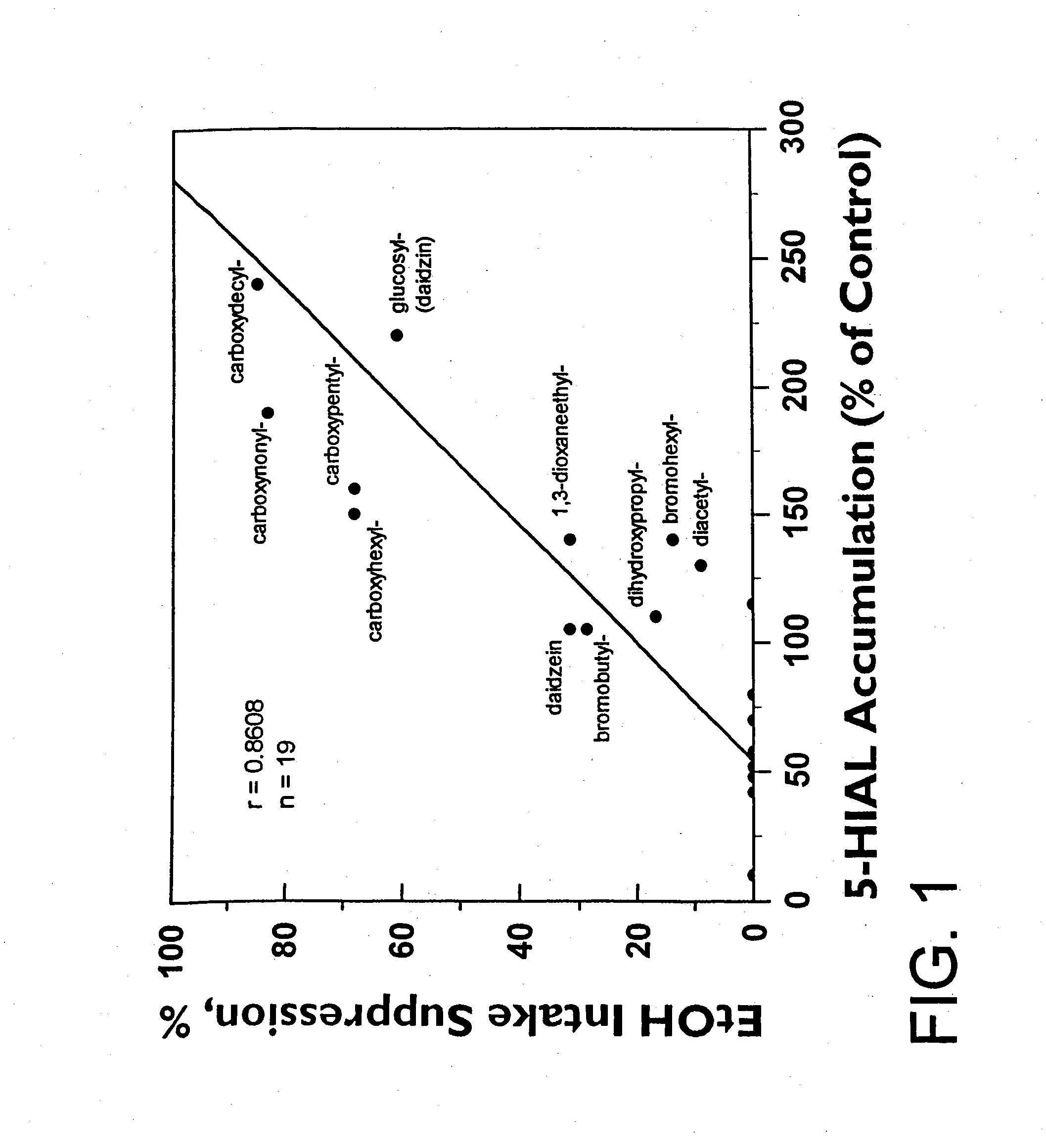
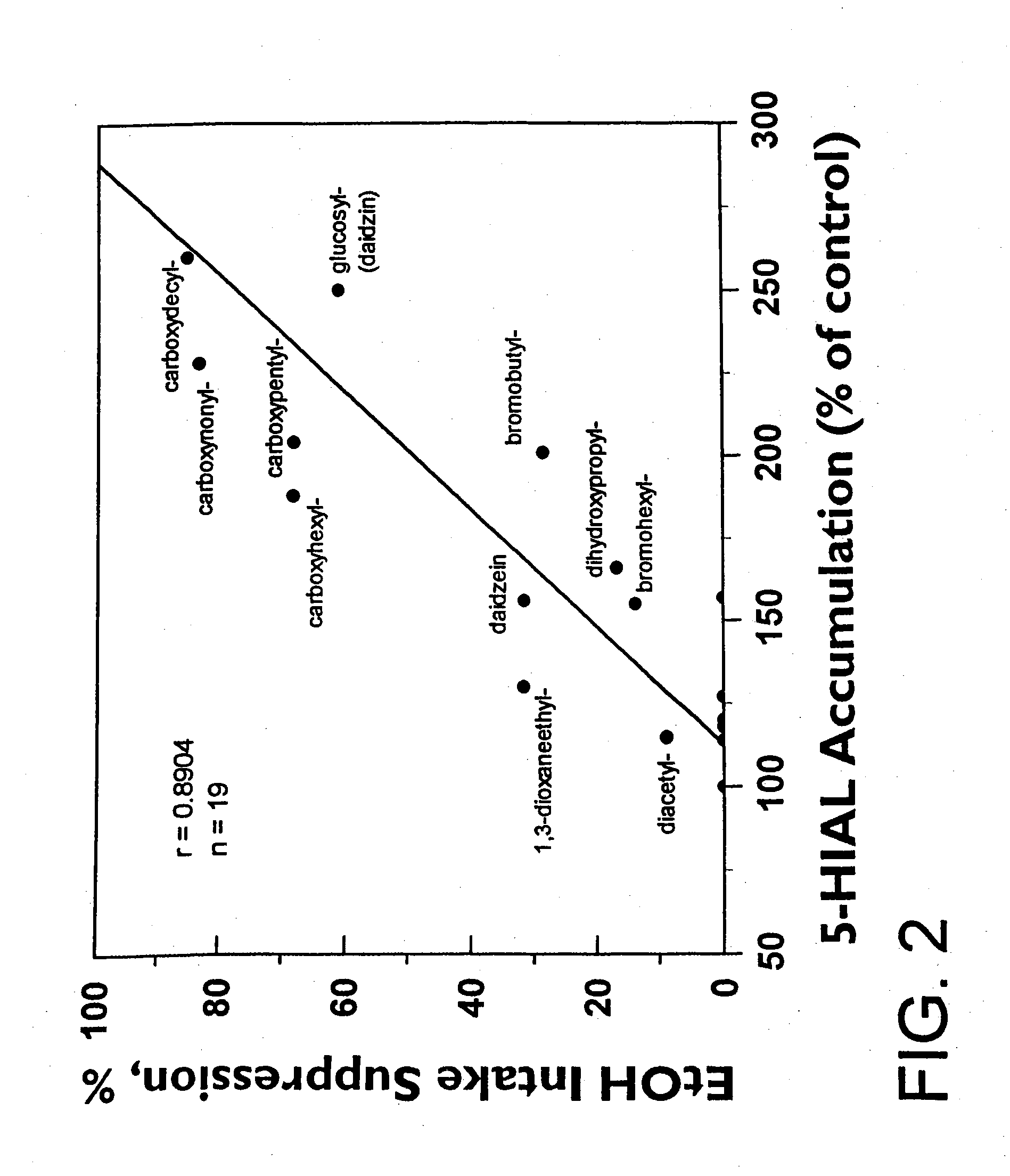
[0053] ALDH activity of daidzin and daidzin analogs was assayed as previously described in U.S. Pat. No. 5,624,910 and Keung, W. M., Klyosov, A. A. & Vallee, B. L. (1997) Proc. Natl. Acad. Sci. USA 94, 1675-1679, such as by monitoring the increase in absorbance at 340 nm due to the formation of NADH (ε340=6.22 mM−1 cm−1) in a Varian Cary 219 spectrophotometer at pH 9.5 when acetaldehyde was used as the substrate, or by monitoring the increase in fluorescence at 430 nm on formation of 6-dimethylamino-2-naphthoic acid (λex=330 nm) in a Perkin-Elmer MPF3 spectrofluorimeter when 6-DMA-2-NA was used as the substrate. Human, hamster, and rat liver mitochondrial and cytosolic ALDH isozymes were purified as described in Klyosov, A. A., Rashkovetsky, L. G., Tahir, M. K. and Keung, W. M., (1996) Biochemistry 35, 4445-4456. Stock solutions of all water soluble substrates and test compounds were made in Milli-Q water and all others were in methanol. The final concentrat...
example iii
Antidipsotropic Activity
[0054] The ethanol intake suppressive activity of daidzin and its structural analogs was determined using alcohol preferring Syrian golden hamsters as previously described. See Keung, W. M. & Vallee, B.L. (1993) Proc. Natl. Acad. Sci. USA, 90, 10008-10012.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com