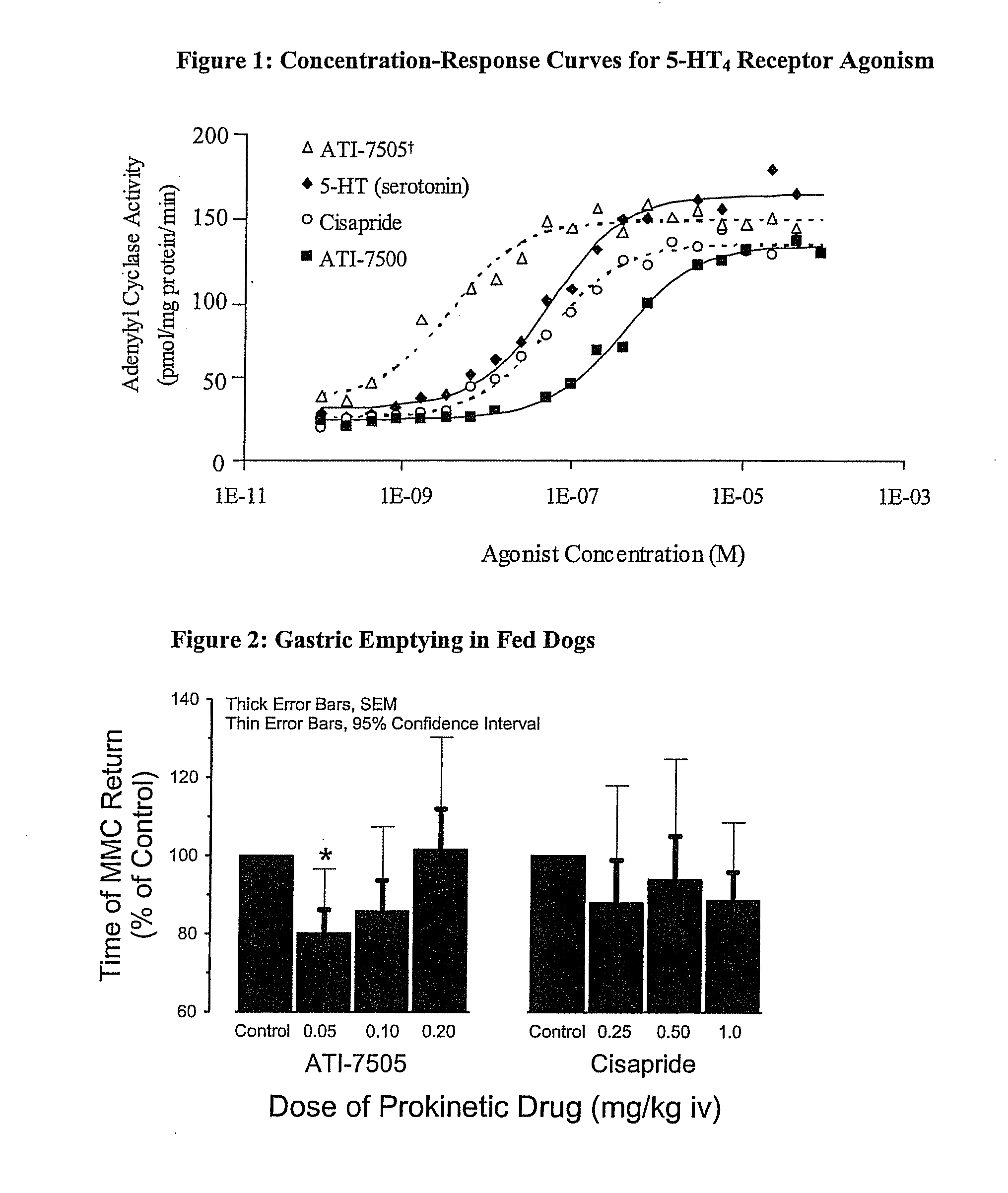
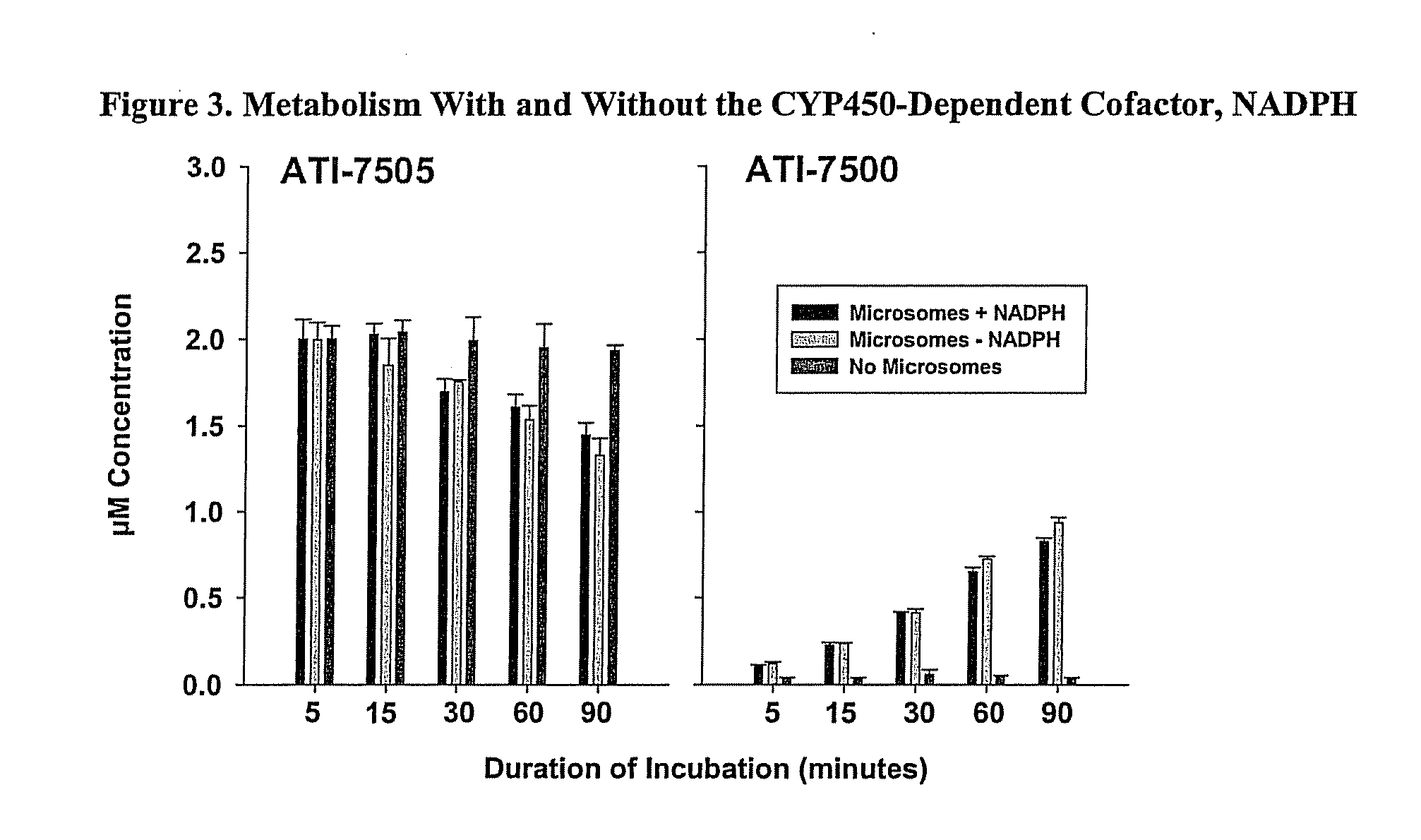
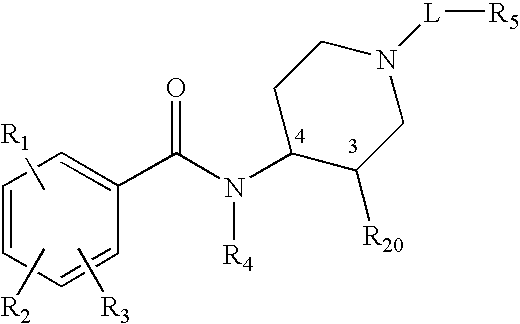
Synthetic Methods and Intermediates for Stereoisomeric Compounds Useful for the Treatment of Gastrointestinal and Central Nervous System Disorders
a technology of stereoisomeric compounds and intermediates, which is applied in the direction of digestive system, drug compositions, organic chemistry, etc., can solve the problems of cisapride administration to a human, failure of the lower esophageal sphincter, nausea and vomiting, etc., and achieve the effect of avoiding the cytochrome p450 drug detoxification system and reducing the incidence of such adverse events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 6-[4R-(4-amino-5-chloro-2-methoxy-benzoylamino)-3S-methoxy-piperidin-1-yl]-hexanoic acid 1-aza-bicyclo[2.2.2]oct-3′R-yl ester, dihydrochloride salt
[0373]
Step 1: Resolution of Racemic Norcisapride
[0374] (−)-2,3-Dibenzoyl-L-tartaric acid ((−)-DBT, about 1 part by weight) was dissolved in ethanol and filtered to remove residual particulates. Separately, racemic norcisapride (about 0.8 part by weight) was dissolved in a mixture of ethanol and water and then filtered. The filtrate was heated to about 75° C. before adding the (−)-DBT solution. After stirring at this temperature for about 30 minutes, the mixture was slowly cooled for several hours to about 5° C. and the product salt was collected under vacuum filtration and washed with EtOH / H2O mixture. The wetcake was recrystallized from EtOH / H2O by heating to about 79° C. and slow cooling to about 5° C. as before. The product was collected on a vacuum filter and washed with EtOH / H2O to give a wetcake.
[0375] The wetcake ...
example 2
[0380] (+) and (−)-norcisapride can be made from its racemic mixture by resolution of the enantiomers using conventional means such as optically resolving acids, according to the method described in U.S. Pat. No. 6,147,093, or in “Enantiomers, Racemates and Resolutions”, by J. Jacques, A. Collet, and S. H. Wilen (Wiley-Interscience, New York, N.Y.), or in S. H. Wilen et al., Tetrahedron (1977) 33:2725.
[0381] The 4 isomers can be obtained in low-mg amounts by using preparative column chromatography followed by evaporation of the solvent. This method is useful for preparing small amounts for analytical and characterization purposes. This is a standard separation method used routinely in analytical labs in order to isolate and characterize metabolites.
[0382] Possible synthetic routes to Compound IV, Compound VI and (+)-Compound II are described below using (+)-norcisapride as a starting material. The routes to Compound III, Compound V and (−)-Compound II are identical except that the...
example 3
Production of (+)-Compound II, Ethyl Ester
[0383] A equimolar mixture of (+)-norcisapride and ethyl 6-bromohexanoate (1 equivalent each), a catalytic amount of KI, and K2CO3 (2 equivalents) in DMF is heated at about 60° C. for several hours or until TLC analysis indicates that the reaction is over. After cooling to room temperature, water is added and the mixture is extracted with EtOAc. The combined organic extracts are washed successively with water, 10% LiCl(aq) solution and brine, then dried over Na2SO4. Concentration gives (+)-compound II, ethyl ester.
[0384] Production of (+)-Compound II
[0385] A mixture of crude (+)-compound II, ethyl ester, from above (1 eq.), KOH (2M, 5 eq.). in MeOH and THF (enough to dissolve) is stirred at room temperature for approximately 1 to 2 hours. The MeOH and THF are removed under vacuum, and the residue is diluted with water. Wash with an organic solvent such as EtOAc. The aqueous layer is acidified to pH ˜5 using HCl. The precipitate is filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com