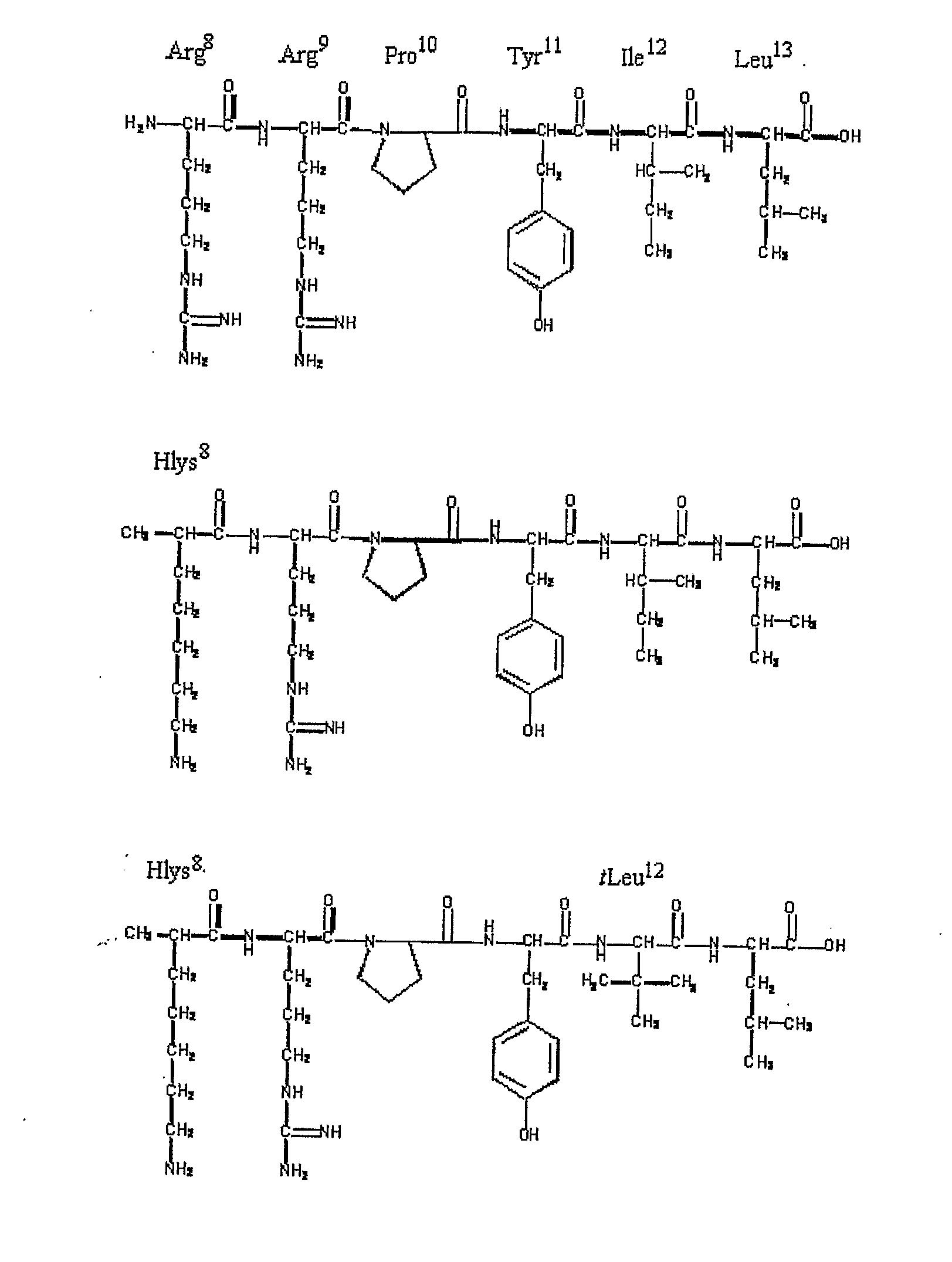
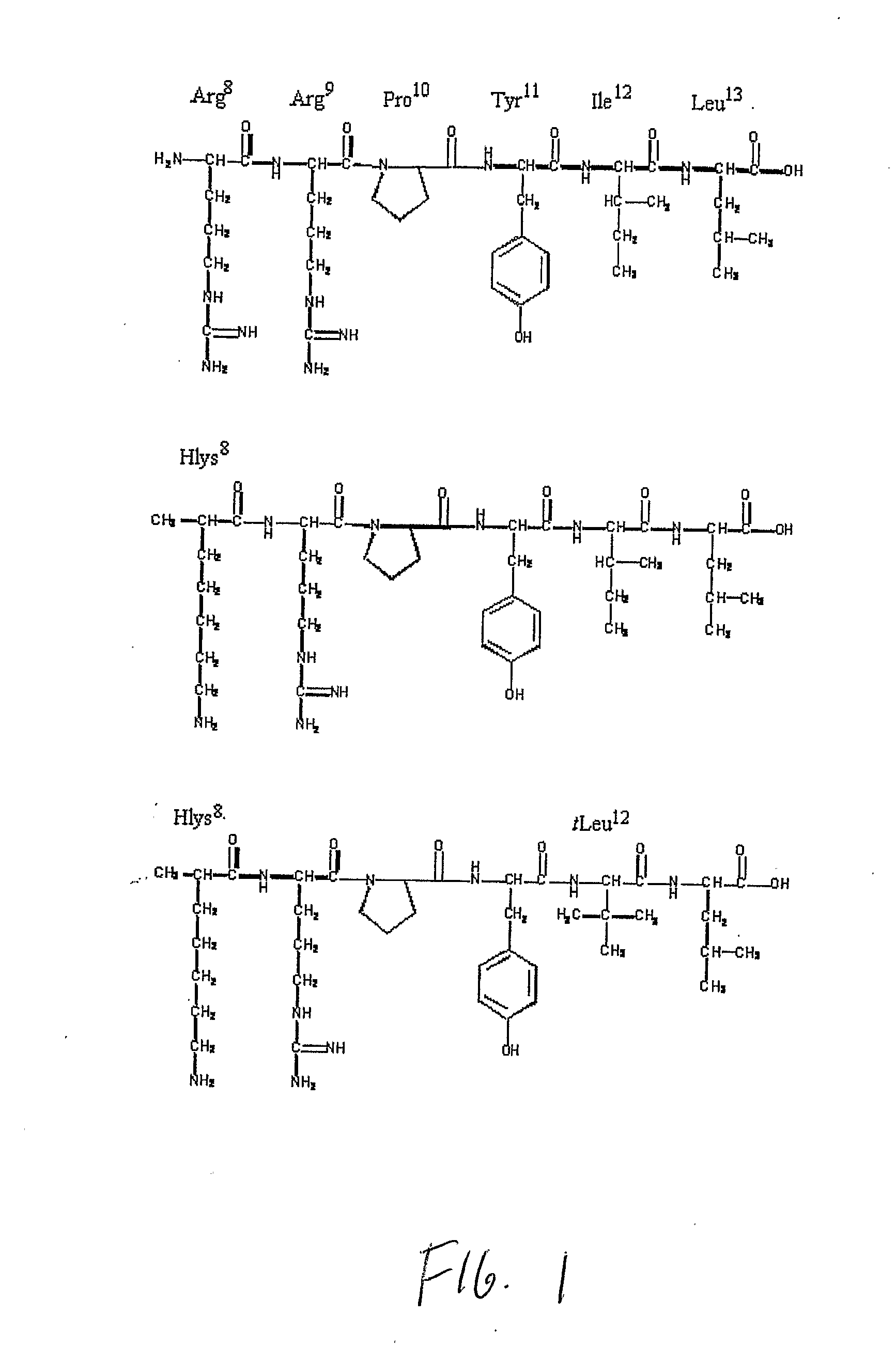
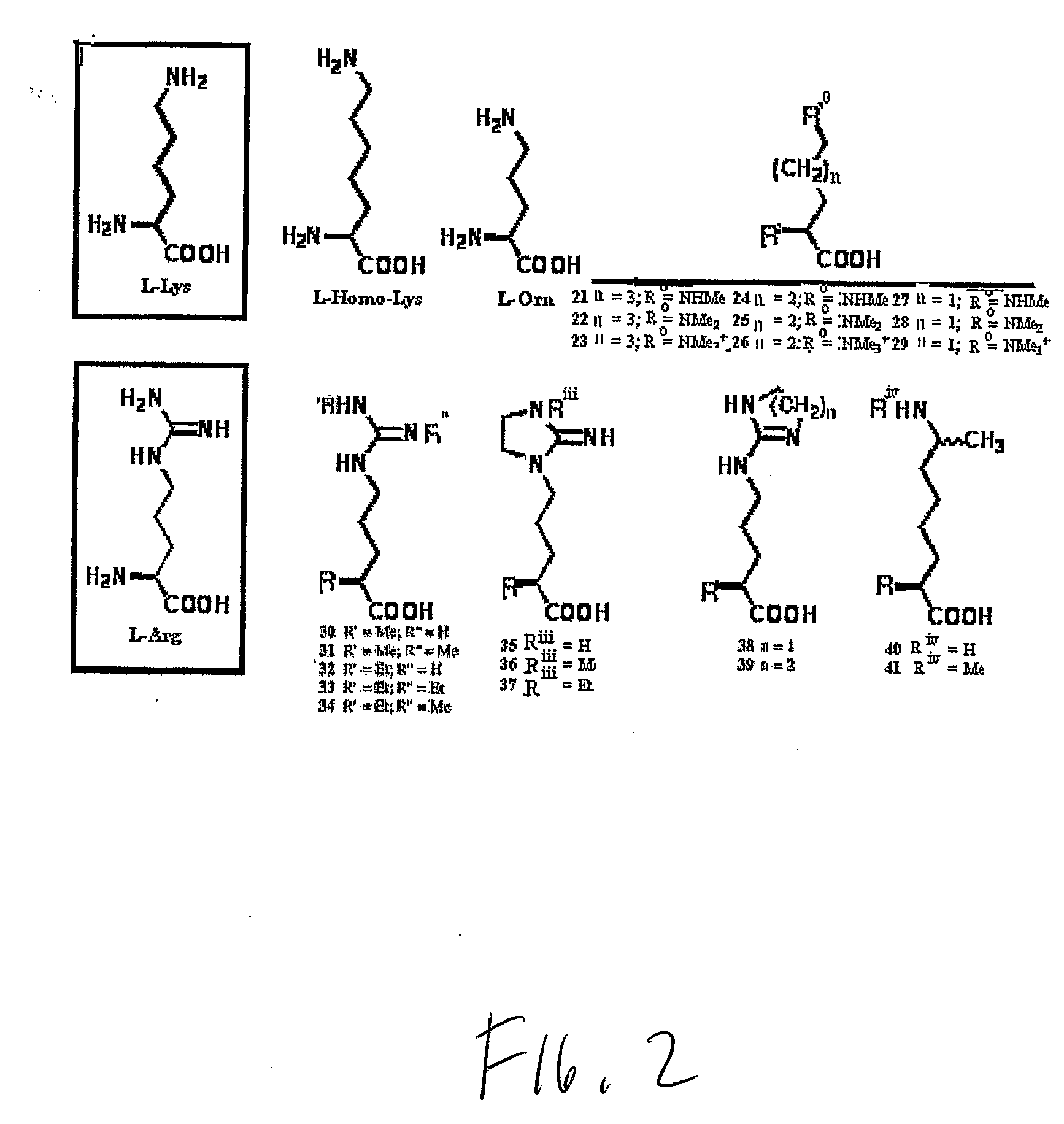
Non-Natural Amino Acids
a technology of amino acids and desamino acids, applied in the direction of depsipeptides, peptide sources, peptide/protein ingredients, etc., can solve the problems of poor drug candidates, unable to cross biological membranes with most peptides, and rarely showing the selectivity required of a viable drug candidate, etc., to achieve selective, long-lasting biological activity, and long-lasting biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
AND PROTOCOLS
[0354]The following examples and protocols are put forth so as to provide those of ordinary skill in the art with a complete disclosure and description of how the compounds claimed herein are made and evaluated, and are intended to be purely exemplary of the invention and are not intended to limit the scope of what the inventors regard as their invention. Efforts have been made to ensure accuracy with respect to numbers (e.g., amounts, temperature, etc.) but some errors and deviations should be accounted for. Unless indicated otherwise, parts are parts by weight, temperature is in ° C. and is at room temperature, and pressure is at or near atmospheric.
[0355]Starting Materials. Solvents are from Fisher Scientific (Pittsburgh, Pa.) and reagents from Aldrich (Milwaukee, Wis.) unless otherwise noted.
[0356]Abbreviations. Trisyl-N3, 2,4,6-triisopropylbenzenesulfonyl azide; Et3N, triethylamine; t-BuCOCl, trimethylacetylchloride; n-BuLi, n-butyl lithium; H2, hydrogen gas; Pd—C,...
example 1
[0357](3(2S),4S)-3-(2-methyl-5-bromo-1-oxovaleryl)-4-(phenylmethyl)-2-oxazolidinone (24a) (FIG. 3). Intermediate 23a was prepared as described previously (57). A solution of 17.4 mL (5 eq) of potassium bis(trimethylsilyl) amide (KHMDS) was added to 100 mL anhydrous tetrahydrofuran (THF) and cooled to −78° C. under positive nitrogen (N2) pressure. A solution of 23a (5.18 g, 15.23 mmol) in 10 mL THF under N2 was cooled to −78° C. and cannulated into the KHMDS solution. This mixture was stirred at −78° C. for 30 min to effect enolate formation. Methyl iodide (CH3I) (1.90 mL, 2 eq) was added to the solution via cannula and stirred at −78° C. for 1 hr at which time the reaction was quenched with 4.09 mL (5 eq) of glacial acetic acid. The solution was warmed to room temperature while stirring over 2 hr and the THF removed in vacuo. The resulting yellow slurry was dissolved in 200 mL half-saturated brine and extracted with CH2Cl2 (3×100 mL). The CH2Cl2 layers were combined, dried over anhy...
example 2
[0358](3(2S),4S)-3-(2-methyl-6-bromo-1-oxohexanyl)-4-(phenylmethyl)-2-oxazolidinone (24b). A slightly modified procedure was used to give 24b. Directly following KHMDS addition to 23b, 5 eq of CH3I was added and the reaction stirred at −78° C. under N2 for 1 hr. Quenching with glacial acetic acid and subsequent extraction and purification protocol was as described above for 24a. Additional silica gel purification eluting with 100% CH2Cl2 gave pure 24b in 10% yield. 1H NMR (400 MHz, CDCl3) δ 7.36-7.19 (m, 5H), 4.72-4.65 (m, 1H), 4.25-4.16 (d J=4.2 Hz, 2H), 3.77-3.67 (m, 1H), 3.46-3.36 (t, J=7.0 Hz, 2H), 3.29-3.22 (dd, J=4.0, 14.0 Hz, 1H), 2.82-2.74 (dd, J=9.0, 14.0 Hz, 1H), 1.92-1.74 (m, 3H), 1.50-1.42 (m, 3H), 1.25-1.21 (d, J=7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 176.9, 153.2, 135.3, 129.6, 129.1, 66.4, 55.6, 38.1, 37.8, 34.1, 32.8, 32.5, 26.1, 18.7.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com