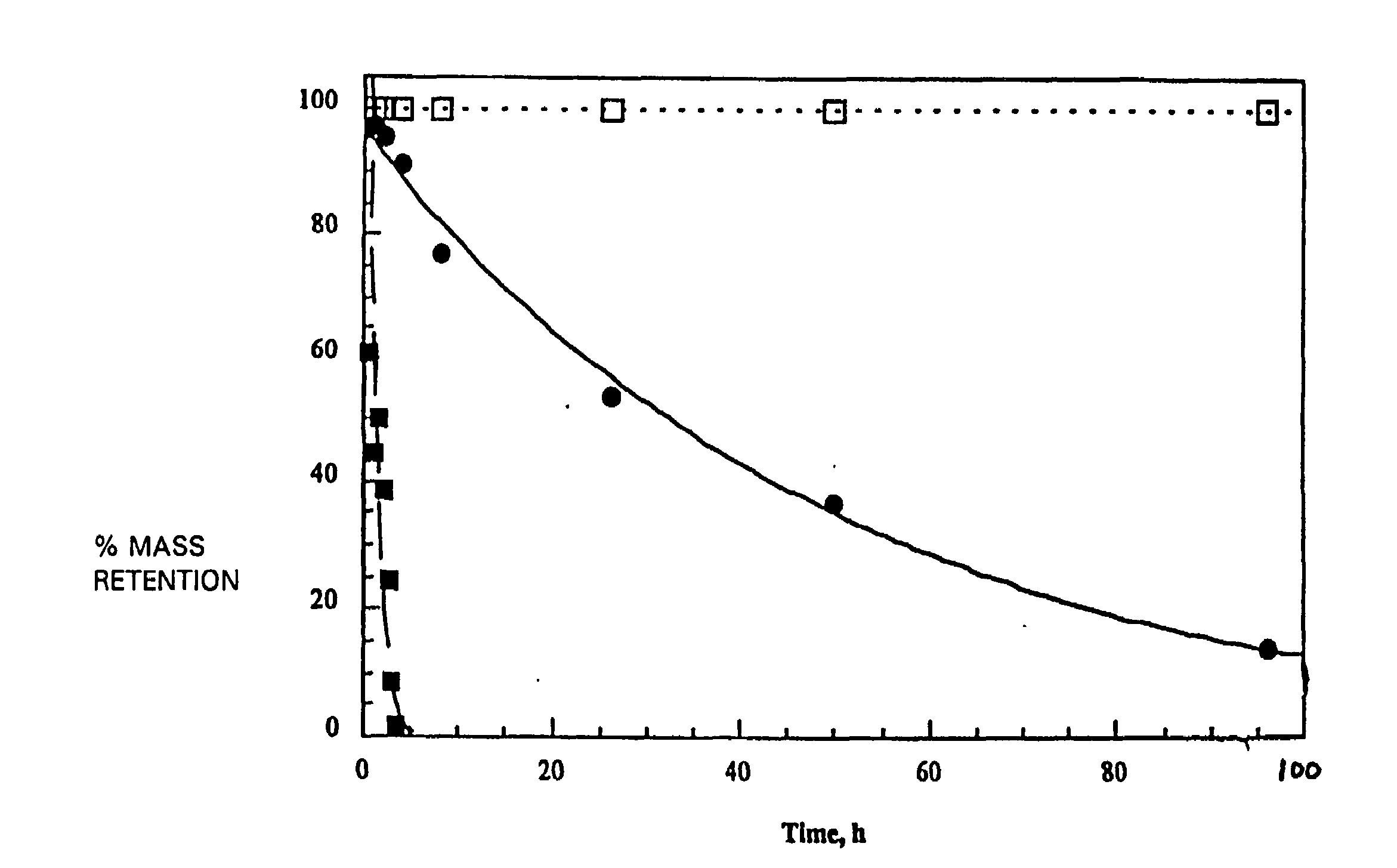
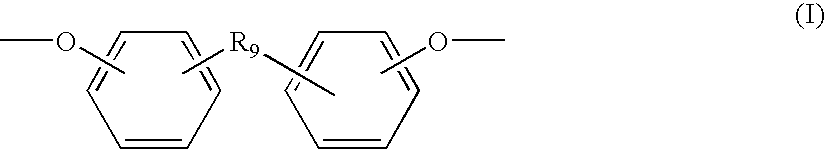
Biodegradable, anionic polymers derived from the amino acid l-tyrosine
a technology of anionic polymers and amino acids, applied in the field of biodegradable anionic polycarbonates and polyarylates having pendent carboxylic acid groups, can solve the problems of limited application of polymer with protected carboxylic acid groups, difficult selective removal of any carboxylic acid protecting groups, and inability to prepare polycarbonates, polyarylates and poly (alkylene oxide) block copolymers thereof, etc., to achieve the effect of preventing the formation of adh
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hydrogenolysis of Poly(DTBn5o-DTEsO Carbonate) Preparation
[0094]In a 500 mL round-bottomed flask was placed 15 g of poly (DTBn-DTE carbonate) which contained DTBn and DTE in a 1:1 ratio. To the flask was then added 150 mL of dry DMF and the mixture was stirred until a clear solution was obtained. To this solution were added 3.5 g of 5 percent Pd on BaSO4 catalyst and 7 mL of 1,4-cyclohexadiene (hydrogen donor). The mixture was stirred at room temperature. A rubber balloon filled with hydrogen gas was attached to the mouth of the flask using a gas inlet adapter. The balloon was replenished with hydrogen as needed. After about 40 h of stirring a 0.5 mL sample was withdrawn, centrifuged, and then precipitated by adding to water with stirring. The precipitate was dried and analyzed by 1H NMR, which showed complete conversion of the benzyl groups to free acid. The reaction was stopped and the reaction mixture was centrifuged. The supernatant was filtered using 0.45 tM syringe filter in s...
example 2
Hydrogenolysis of Poly(DTBn0.05-DTE0.95 Carbonate)
Preparation
[0098]The hydrogenolysis of a 15 gram sample of poly (DTBn-DTE carbonate), which contained DTBn and DTE in a 1:19 ratio and had a Mw of 286 Kda and Mn of 116 Kda was performed as in Example 1.
Structure Proof
[0099]The 1H NMR spectrum of the product in DMSO-d6 showed the following resonances (δ, ppm relative to TMS): 8.40 (br s, 0.95H, NH of DTE), 8.25 (br s, 0.05H, NH of DT), 7.15-7.35 (m, 8H, aromatic H's), 4.71 (m, 1H, CH of tyrosine), 4.03 (q, 1.9H, CH2—CH3), 2.1-3.3 (m, 6H, CH2's of DAT and Tyrosine), 1.11 (t, 2.85H, CH2—CH3). Also a multiplet that is found in poly (DTBn-DTE carbonate) at 5.1 ppm due to benzyl H's was completely absent indicating complete removal of the benzyl protecting groups. The 1:19 ratio of the DT-NH peak to the DTE-NH peak indicates that the polymer is made of 5% DT and 95% DTE. The ratio of CH group to the ethyl ester group shows that there are nineteen ethyl ester groups for every twenty monome...
example 3
Hydrogenolysis of Poly(DTBn0.025-DTE0.90 Carbonate)
Preparation
[0101]Hydrogenolysis of a 15 g sample of poly (DTBn-DTE carbonate) which contained DTBn and DTE in a 1:9 ratio and had a Mw of 183 Kda and Mn of 84 Kda was performed as in Example 1.
Structure Proof
[0102]The 1H NMR spectrum of the product in DMSO-d6 showed the following resonances (δ, ppm relative to TMS): 8.40 (br s, 0.9H, NH of DTE), 8.25 (br, s, 0.1H, NH of DT), 7.15-7.35 (m, 8H, aromatic H's), 4.50 (m, 1H, CH of tyrosine), 4.03 (q, 1.8H, CH2, CH3), 2.1-3.3 (m, 6H, CH2's of DAT and Tyrosine), 1.11 (t, 2.7H, CH2, —CH3). Also a multiplet that is found in poly (DTBn-DTE carbonate) at 5.1 ppm due to benzyl H's was completely absent indicating complete removal of the benzyl protecting groups. The 1:9 ratio of the DT-NH peak to the DTE-NH peak ester group shows that there are nine ethyl ester groups for every ten monomer subunits. These spectral data indicate that the polymer contains DT and DTE in 1:9 ratio and the benzyl pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
| Adhesion strength | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com