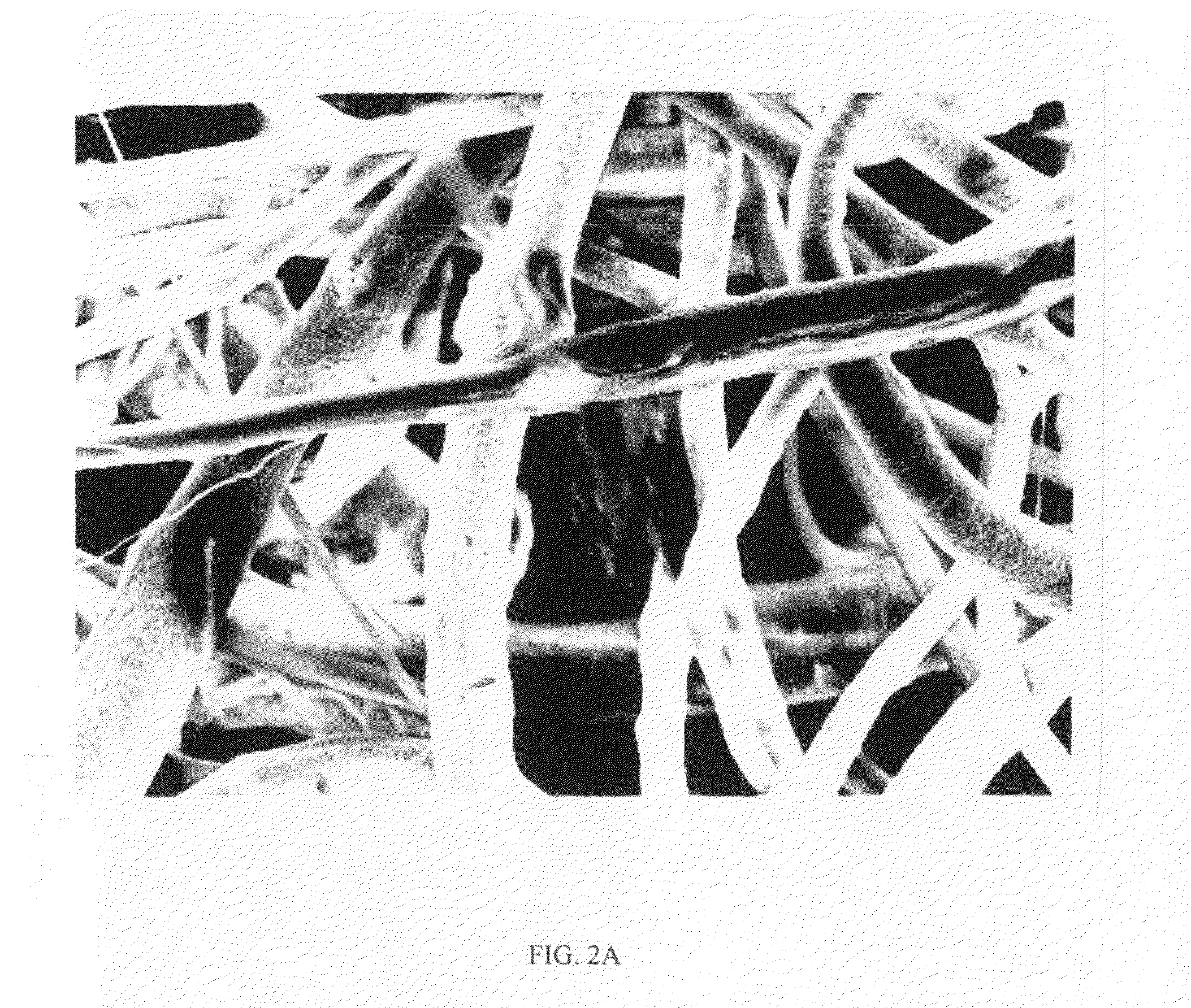Electrospun Ceramic-Polymer Composite As A Scaffold For Tissue Repair
a technology of electrospun fibers and composites, which is applied in the field of three-dimensional matrix preparation of micron-sized electrospun fibers, can solve the problems of large bone defects, limited clinical use of ceramics, and high risk of disease transmission, and achieve the effect of facilitating bone repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Fabrication of Tissue Engineering Scaffolds
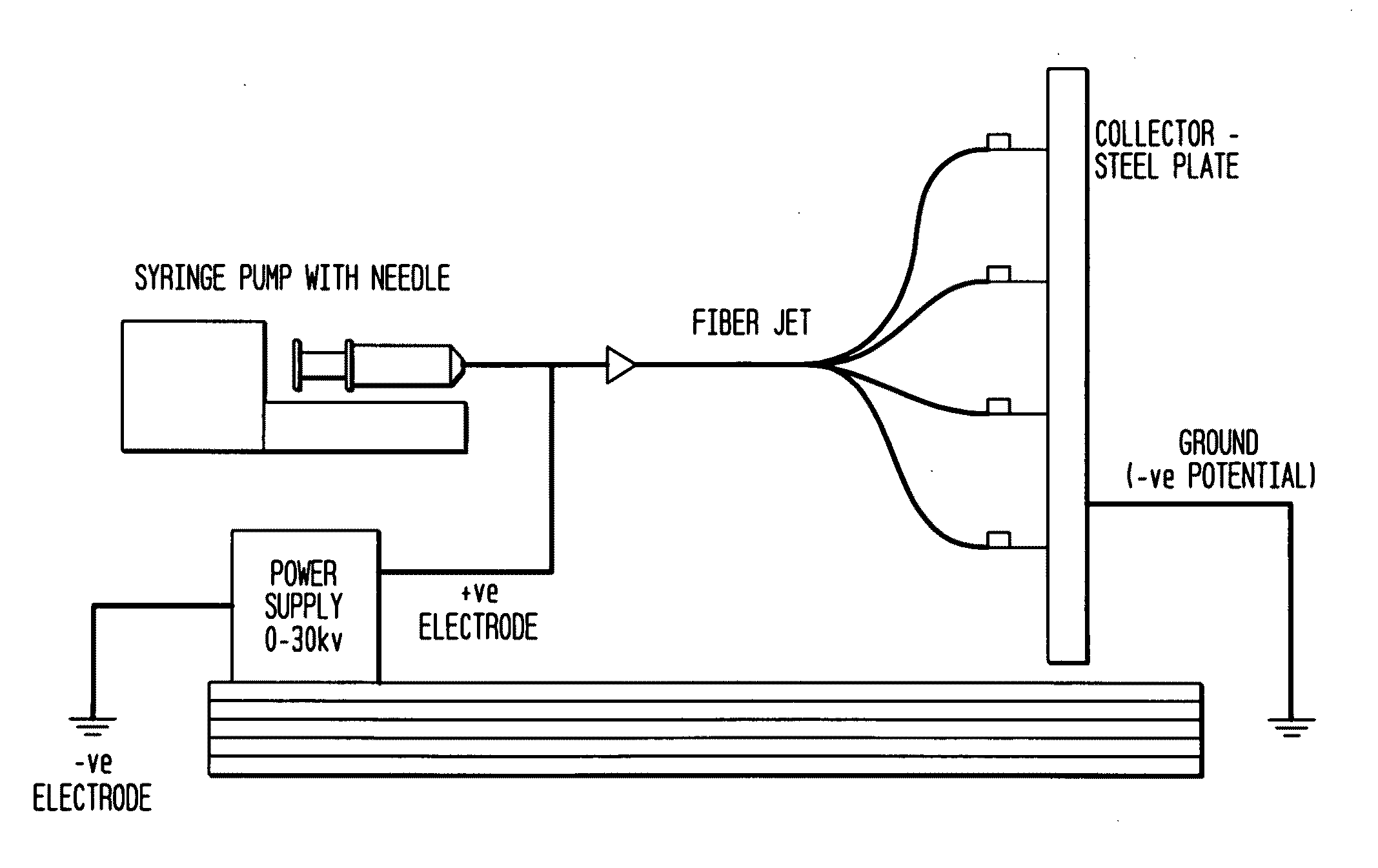
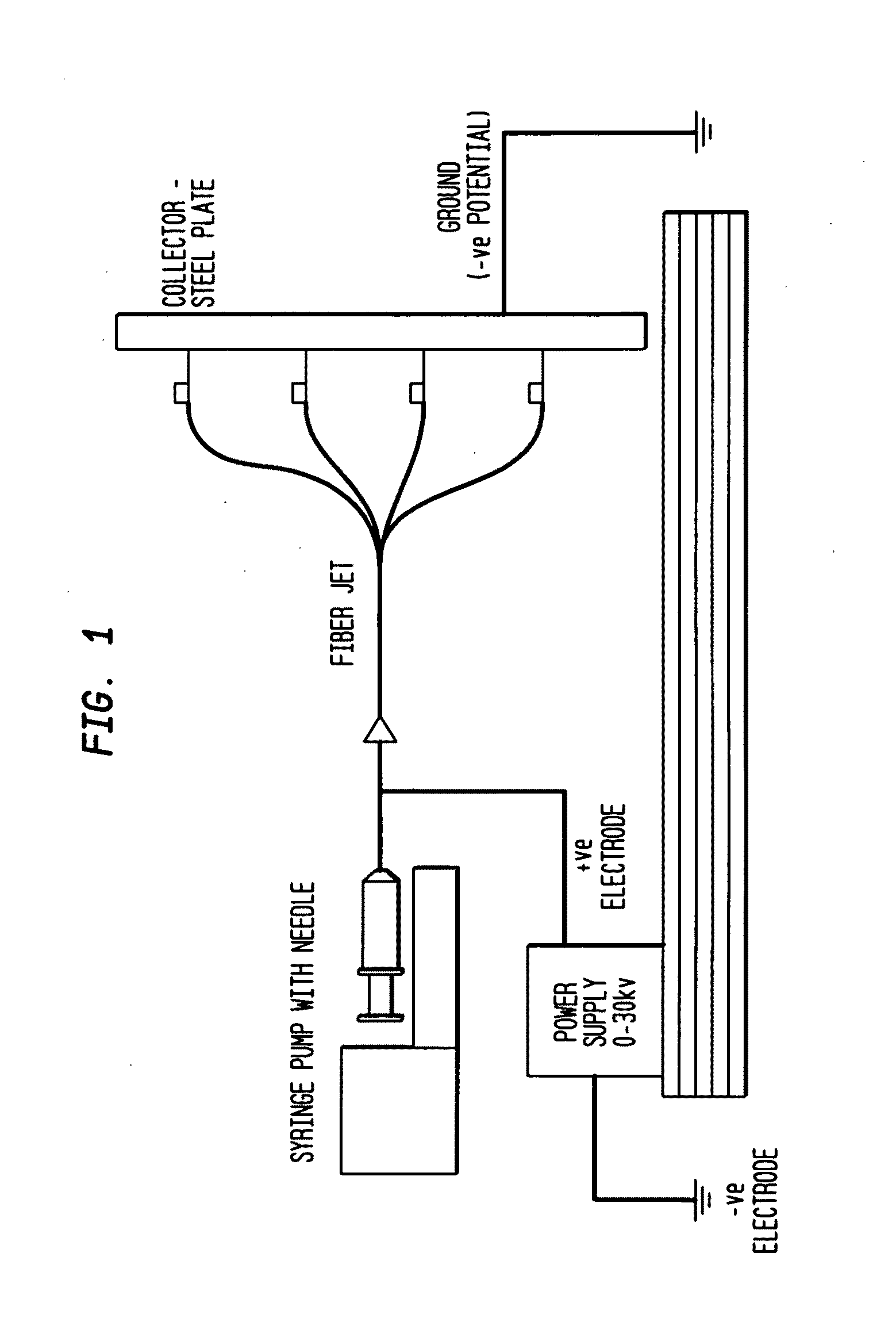
[0057]The Electrospinning Process
[0058]Electrospinning is a fiber forming technique that relies on charge separation to produce nano- to microscale fibers, which typically form a non-woven matrix. The terms “nonwoven matrix”, “nonwoven mesh” or “nonwoven scaffold” are used interchangeably herein to refer to a material comprising a randomly interlaced fibrous web of fibers. Generally, individual electrospun fibers have large surface-to-volume and high aspect ratios resulting from the smallness of their diameters. These beneficial properties of the individual fibers are further enhanced by the porous structure of the non-woven fabric, which allows for cell infiltration, cell aggregation, and tissue formation.
[0059]The electrospinning process is affected by varying the electric potential, flow rate, solution concentration, capillary-collector distance, diameter of the needle, and ambient parameters like temperature. Therefore, it is possib...
example 2
Incorporation of Stem Cells into a Ceramic-Polymer Matrix of the Present Invention
[0102]In some embodiments, osteoblasts or mesenchymal stem cells are incorporated into the ceramic-polymer scaffold. In some such embodiments, the electrospun ceramic-polymer matrix is a scaffold for tissue engineering in vivo. In some such embodiments, the electrospun ceramic-polymer matrix provides a culturing medium in vitro.
[0103]Cell Proliferation
[0104]Human MSCs are isolated from adult, human whole bone marrow according to standard techniques and are seeded onto the ceramic-polymer scaffolds of the present invention grown in standard growth medium (DMEM, 10% fetal bovine serum, 1% antibiotic / antimycotic) for 14 days. Cell proliferation is assessed using Vybrant's MTT Cell Proliferation Assay Kit (Molecular Probes, Inc.).
[0105]Osteogenic Differentiation.
[0106]Bioactive ceramic-polymer scaffolds are created by the process of electrospinning, and human mesenchymal stem cells are grown on the scaffol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore sizes | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com