Azole compounds used as tuberculostatic and leishmanicide agents
a technology of azole compounds and leishmanicides, which is applied in the field of azole compounds used as tuberculosis and leishmanicide agents, can solve the problems of insufficient or even non-existent treatment of infectious and parasitic diseases, insufficient government funding to fight these kinds of diseases, and thousands of deaths or serious damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
A—Pharmacological Evaluation of the Triazole Derivatives of Type I (Formula IX) and II (Formula X)
[0104]
A.1—Antimicrobial Activity
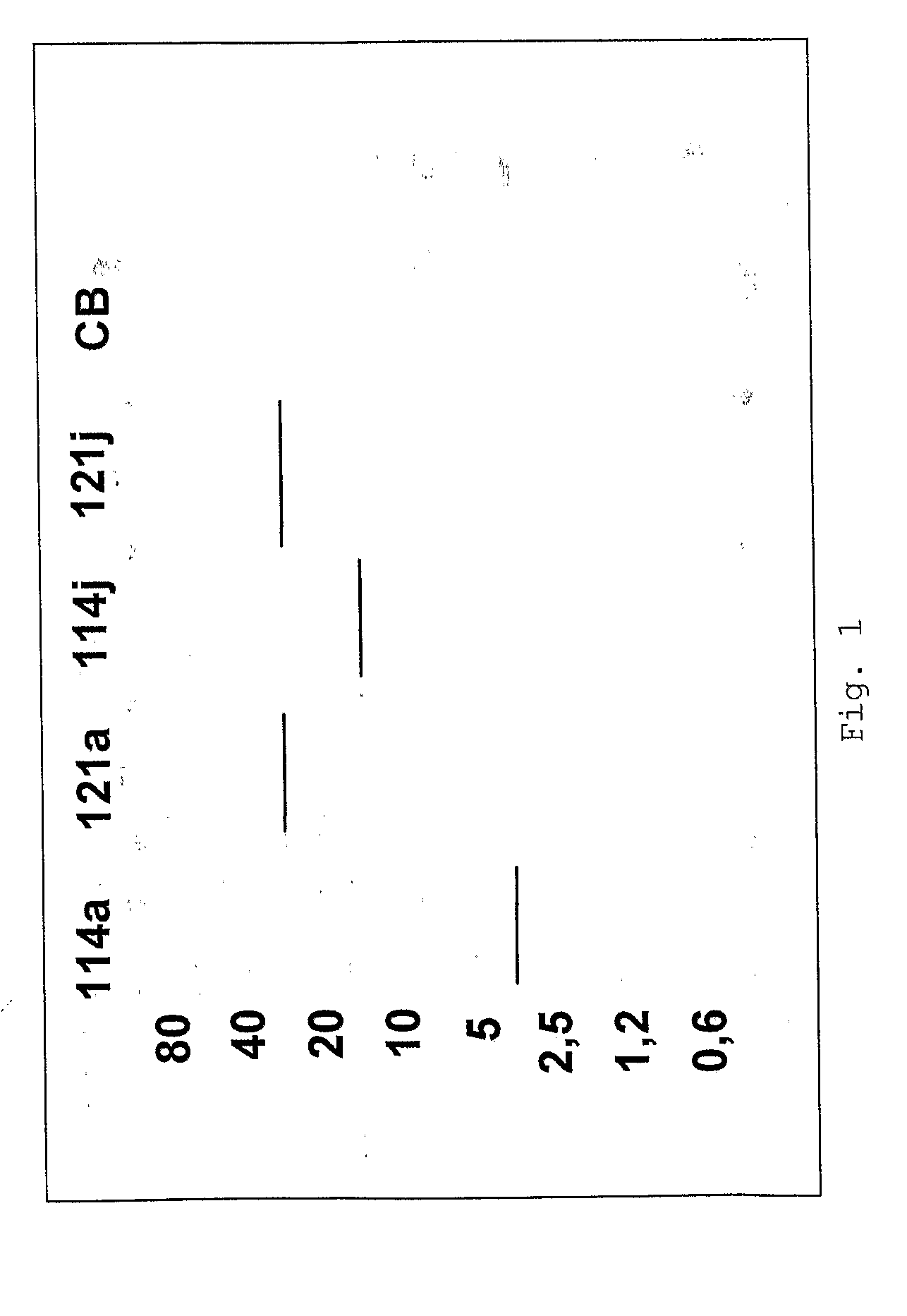
[0105]Type I and II 1,2,3-triazole derivatives were submitted to a primary biological evaluation, in vitro, regarding the inhibitory activity of the Mycobacterium tuberculosis H37Rv (ATCC-27294).
[0106]The definition of minimum inhibitory concentrations (MIC) of substances, that is, the smallest concentration of the compound in which bacterial growth is not observed, was made using the calorimetric method known as MABA (Microplate Alamar Blue Assay). This method consists in an essay performed by the micro dilution in plates, using, as cell growth indicator, the Alamar Blue® indicator pigment, which is a fluorescent / colorimetric indicator with redox property. The oxidized form is blue (non-fluorescent) and indicates the absence of bacterial growth. The reduced form presents a pink color (fluorescent), indicates the proliferation of bacteria. This methodolog...
example 2
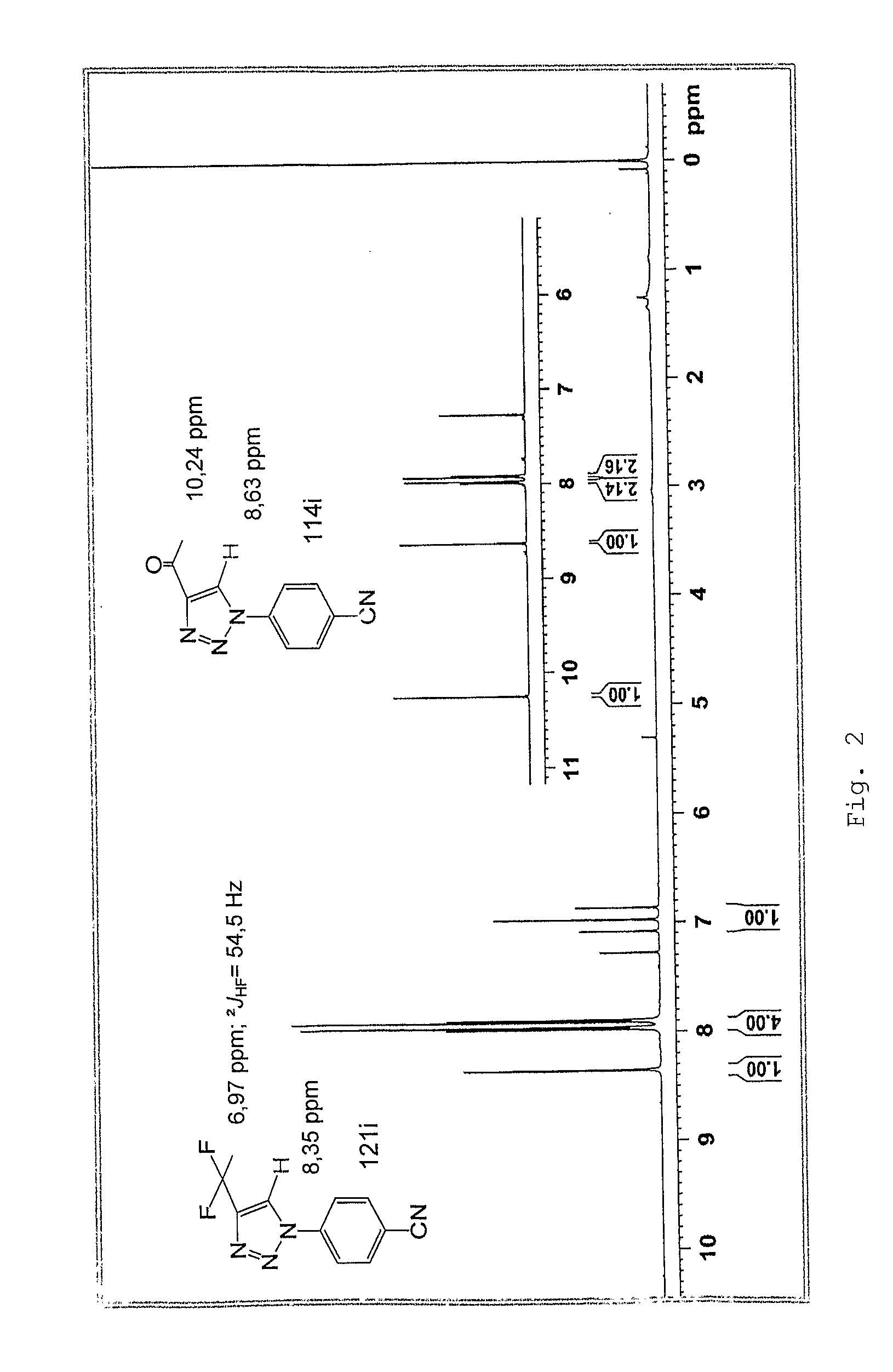
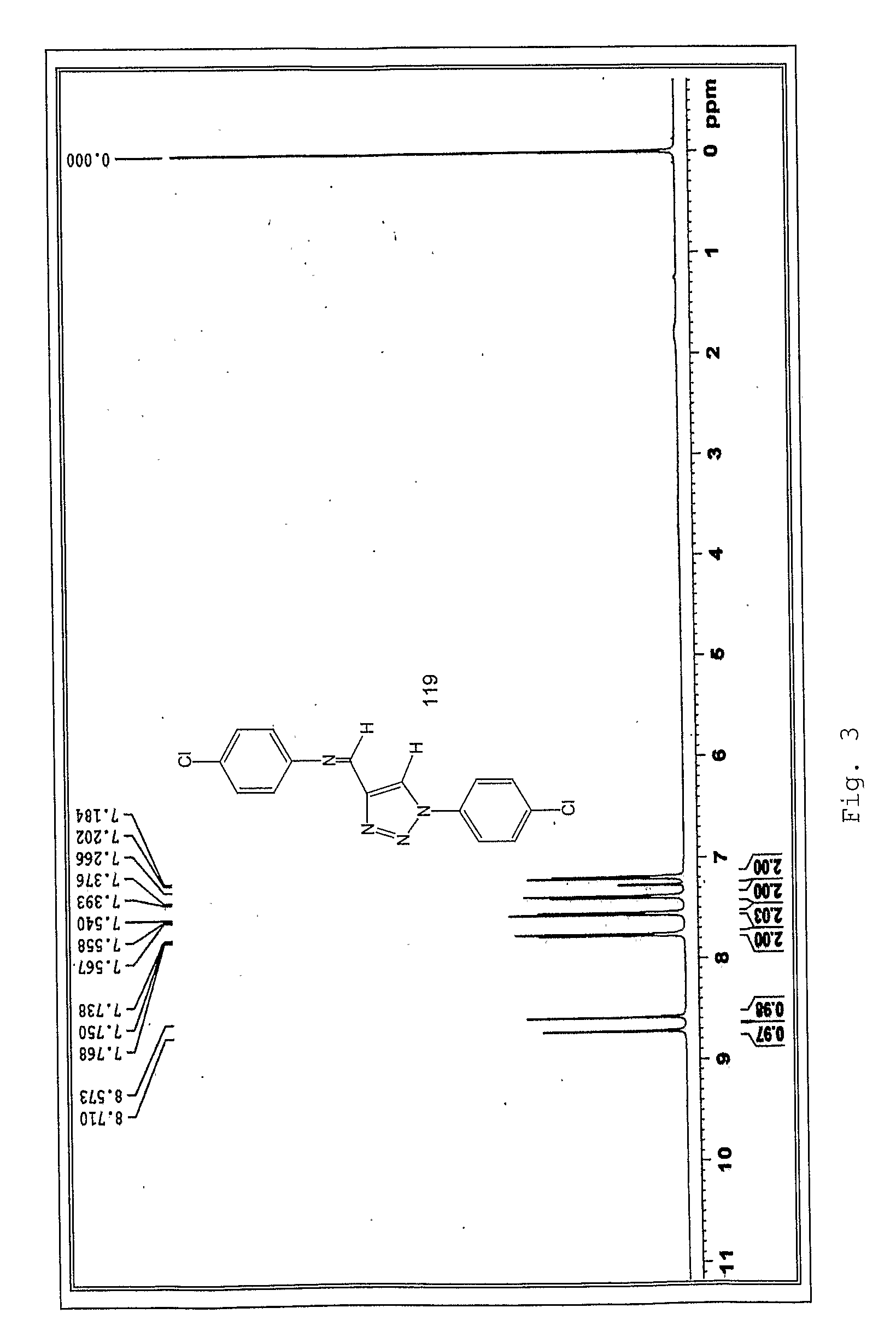
[0120]Below there is the detailed description of the obtainment of both type I and II 1,2,3-triazole compounds, which were confirmed by analytical methods represented by FIG. II.
A—Method of General Obtainment of Type I 1,2,3-triazole Derivatives:
[0121]
[0122]In a balloon containing 5 mmol of diazomalonaldehyde 30.0 mL of distilled water was added. Then, a recently prepared solution with 4.5 mmol of the chloride derivative of the desired amine was added slowly into 5 mL of distilled water. The reaction mixture was under disturbance at room temperature, for 4 hours, and the precipitation of triazole product was observed. The solid was insulated by filtration and washed with ice water.
[0123]This methodology was used to obtain the below mentioned compounds:
a—4-carboxaldehyde-1-(4-chlorophenyl)-1H-1,2,3-triazole (114a)
[0124]The derivate (114a) was prepared with 75.0% of output, as of the reaction of the diazomalonaldehyde with the 4-chloroaniline chloride, thus obtaining an amorphous whit...
example 3
A—Pharmacological Analysis of Type I (Formula XII) and II (Formula XIII) Imidazole Derivatives
[0365]
A.1—Antimicrobial Activity
[0366]The imidazole derivatives with general formula XII and XIII were submitted to a primary biological evaluation, in vitro, regarding the inhibitory activity of Mycobacterium tuberculosis H37Rv (ATCC-27294).
[0367]The assessment of the minimum inhibitory (MIC) of the substances, that is, the smallest concentration of the compound where the bacterial growth is not observed, was made using the calorimetric method known as MABA (Microplate Alamar Blue Assay). This method consists in an essay performed by the micro dilution in plates, using, as cell growth indicator, the Alamar Blue® indicator pigment, which is a fluorescent / colorimetric indicator with redox property. The oxidized form is blue (non-fluorescent) and indicates the absence of bacterial growth. The reduced form presents a pink color (fluorescent), indicates the proliferation of bacteria.
[0368]To pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com