Mucosal Bioadhesive SLow Release Carrier for Delivering Active Principles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
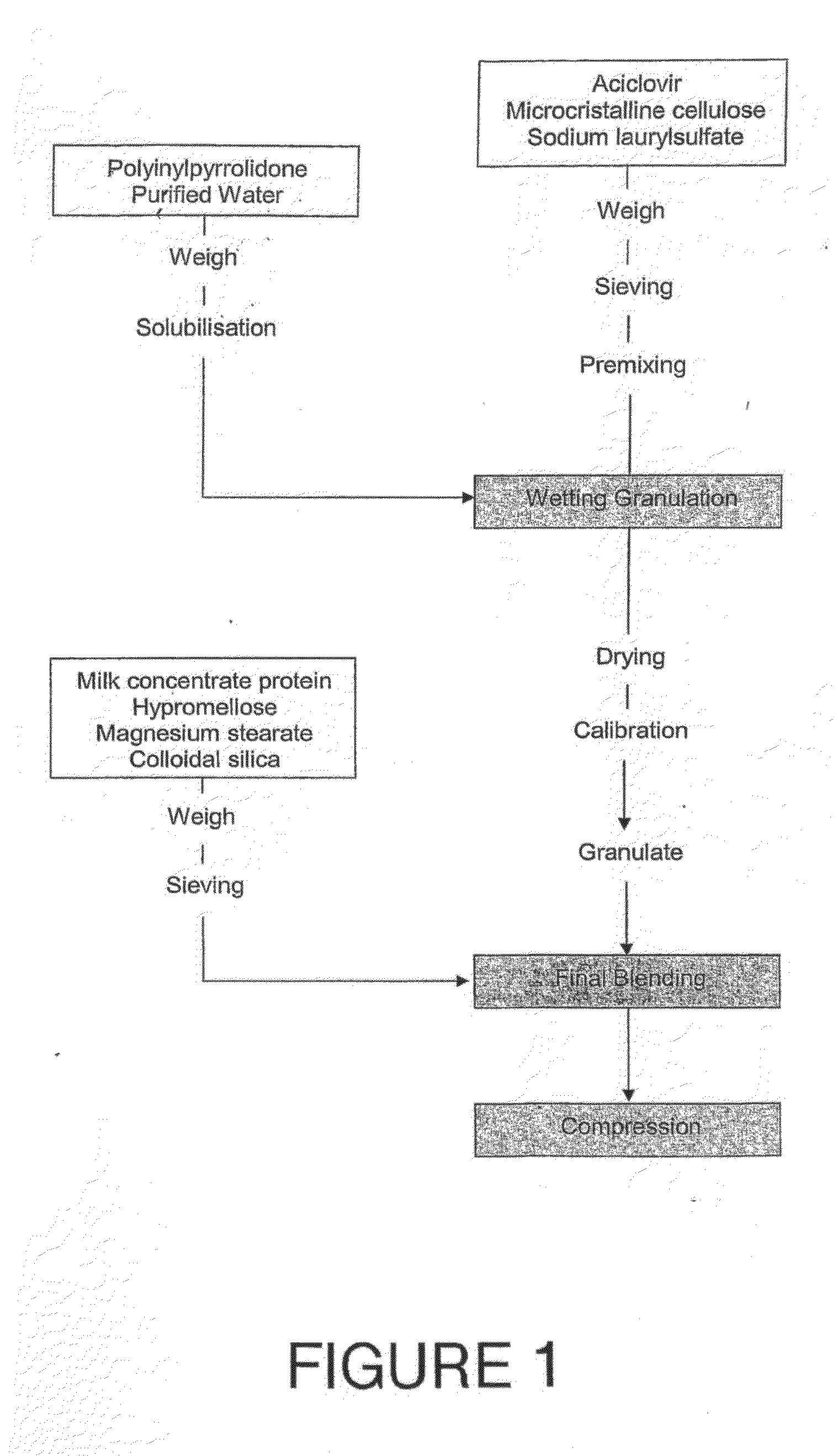
Preparation of the Bioadhesive Slow Release Carrier of the Present Invention
[0128]100 mg of acyclovir, 15% by weight microcrystalline cellulose and 45% by weight of sodium lauryl sulfate were weighed and sieved with a 0.7 to 2 mm sieve before premixing in a blender to provide the “initial mix.”
[0129]At the same time, 0.4% by weight polyvinylpyrrolidone was dissolved in purified water: The resulting solution was added to the initial mix and further stirred. The wetted mixture was then granulated using a pharmaceutical mixer or granulator such as a planetary mixer or high sear mixer and dried and then calibrated to 500 μm. The resulting pellets formed the “primary granules.”
[0130]20% milk concentrate protein, 15% hypromellose, 1% magnesium stearate and 0.4% of colloidal silica were weighed and sieved using a 500 μm sieve. These ingredients were then added to the primary granulation to form the “final blending” mixture. The final blending mixture was then compressed using a tablet pres...
example 2
Particle Size Distribution of the Primary Granules
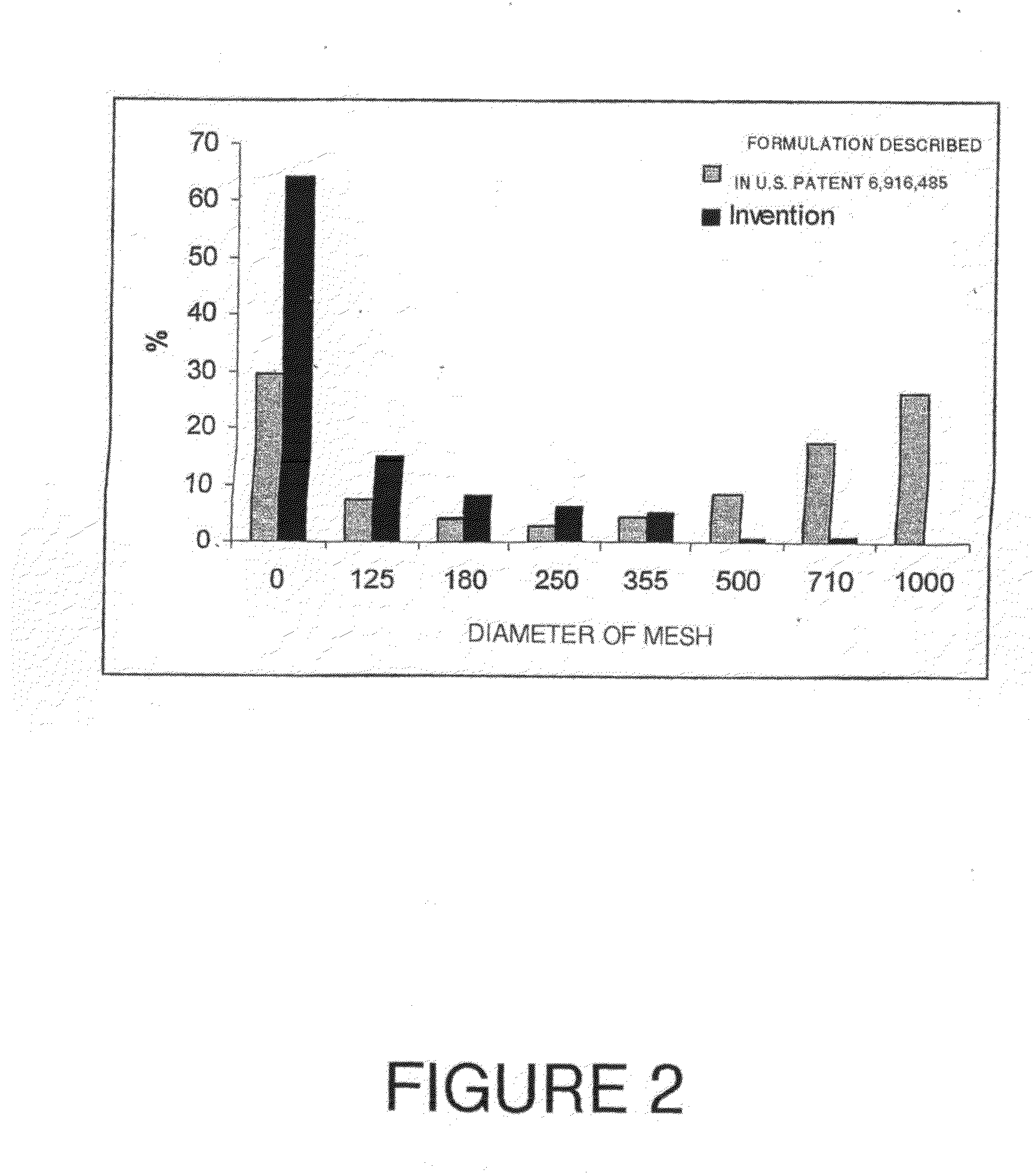
[0133]The primary granules obtained from the procedure in Example 1, after granulating, drying and calibrating, were then evaluated by sieve analysis based on the procedure described in the European Pharmacopoeia. Also an analysis was undertaken to determine the content of acyclovir in the different fractions of the granulations or in the whole granule. The acyclovir content in the granule was assayed using phosphate buffer at pH 6. Two different formulations were compared. The first formulation corresponded to those obtained in Example 1 of the present invention, while the “control formulation” was that disclosed in U.S. Pat. No. 6,916,485, to obtain the “primary grain” set forth in FIG. 1 therein.
[0134]The results are presented in FIG. 2. When granulations were prepared according to the formulation described in U.S. Pat. No. 6,916,485 (white bars), the size distribution of the granules reveals a strong heterogeneity, with the prese...
example 3
In Vitro Evaluation of an Acyclovir Bioadhesive Slow Release Carrier
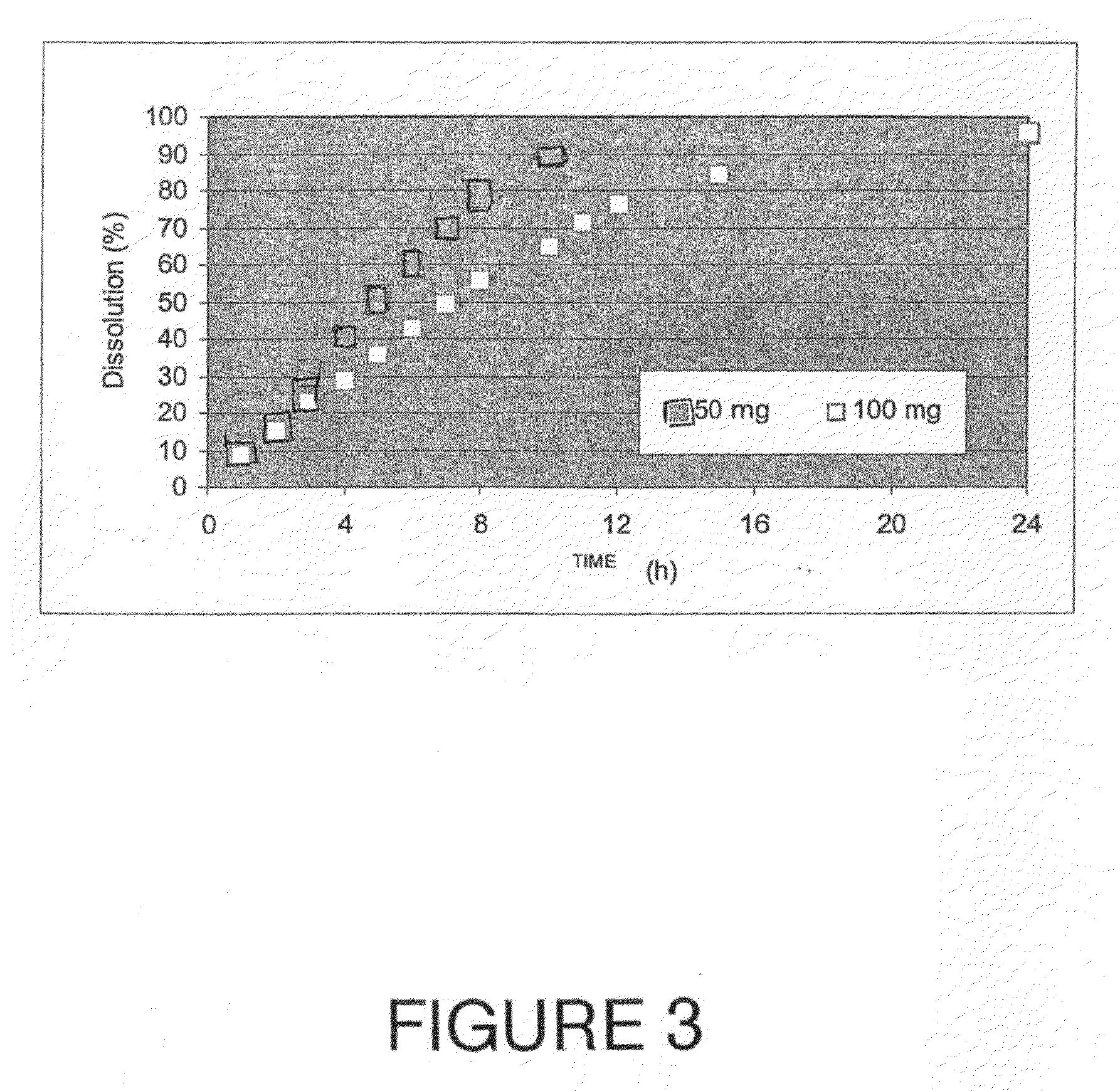
[0136]The bioadhesive slow release carriers obtained by the process described in Example 1 were tested for acyclovir release through a dissolution method. The test was conducted according to the current dissolution testing described in U.S. Pharmacopoeia, 23rd edition, Chapter 711 (Dissolution) pages 1791-1793. More specifically, sample vessels were submerged in a water bath at 37° C., in a suitable dissolution medium of phosphate buffer at a pH of 6 and the content of the vessels were agitated using a “rotating basket” attached to a shaft that is also attached to another shaft. The solid bioadhesive slow release carriers of the present invention were placed in the medium filled vessel at time zero. The water bath was maintained at 37° C. throughout the experiment, as well as the mixing speed of 60 rpm. 1 ml aliquots were taken from the vessels every hour for the first eight hours, then at 10, 11, 12, 15 and 24 hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com