Compositions and methods for treating lysosomal storage disease
a lysosomal storage disease and composition technology, applied in the direction of drug compositions, peptide/protein ingredients, metabolic disorders, etc., can solve the problems of lack of effective treatment currently available for this disease, loss of organ function and death, and inability to demonstrate the biochemical and clinical effectiveness of enzyme replacement in fabry disease, as well as other lysosomal storage diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Vector Construction
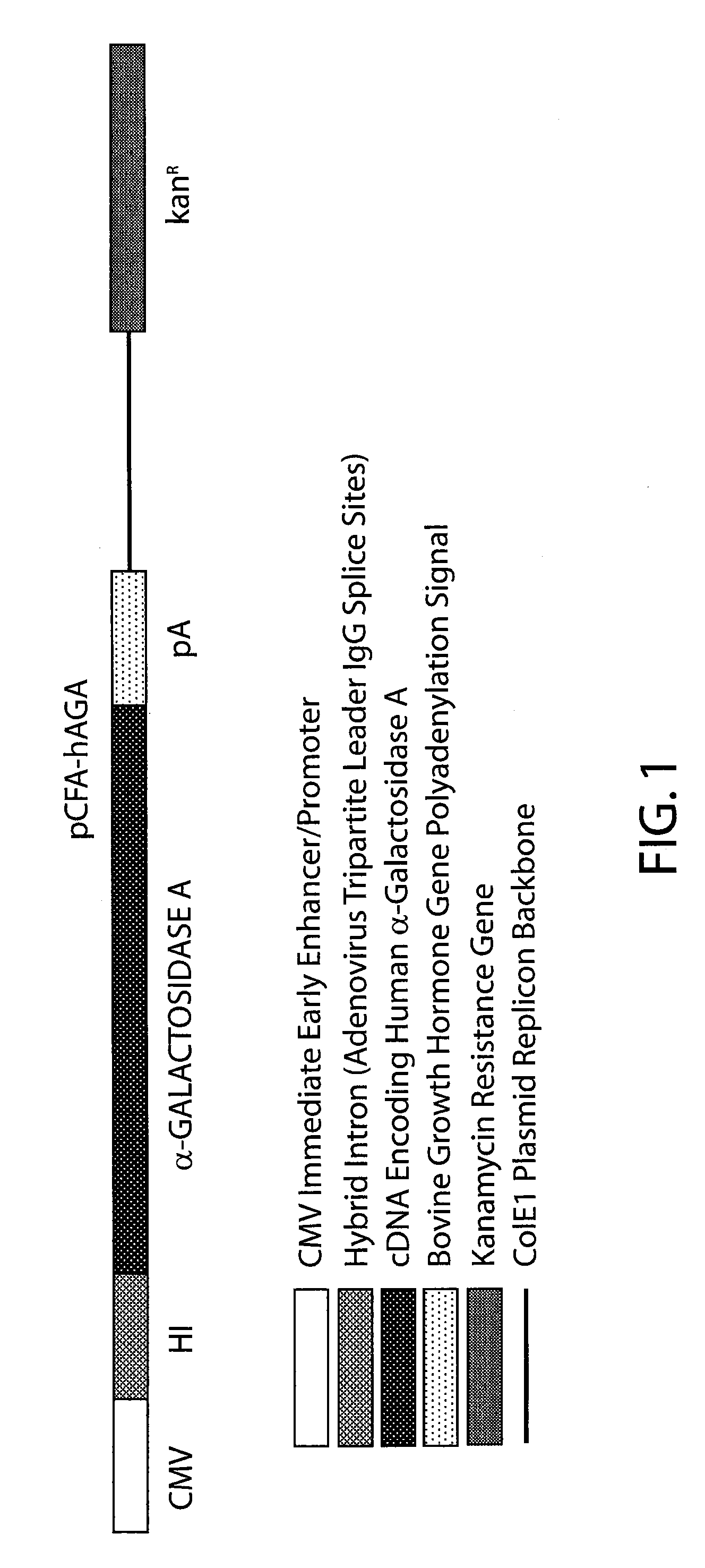
[0065]pCFA-hAGA
[0066]This plasmid expression vector utilizes the cytomegalovirus immediate early promoter to drive expression of the human α-galactosidase A cDNA. A hybrid intron was included after the promoter to provide splice sites to enhance expression. The polyadenylation signal was taken from the bovine growth hormone gene. The ColE1 replicon from pUC was used as a backbone for replication in E. coli. The kanamycin resistance gene was used to select for plasmid maintenance. The construction of the pCFA-hAGA is analogous to the construction of the pCFI vector containing a CFTR transgene disclosed, e.g., in U.S. Pat. No. 5,783,565, the disclosure of which is incorporated herein by reference. In the pCFA-hAGA vector, an α-galactosidase A transgene is substituted for the CFTR transgene in pCFI.
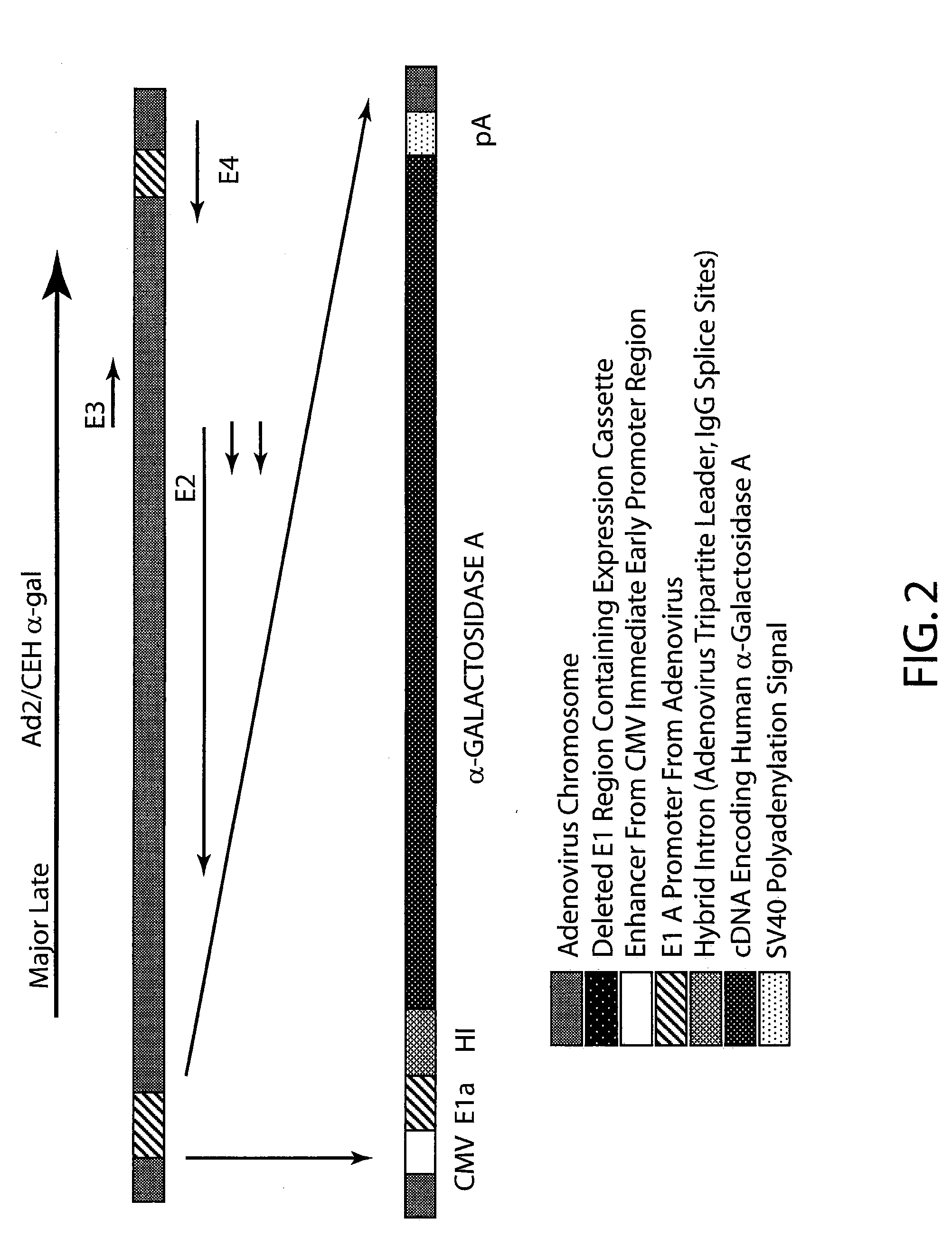
Ad2 / CEHα-gal
[0067]The E1-deleted adenovirus expression vector using an Ad2 serotype viral backbone was constructed as provided in U.S. Pat. No. 5,670,488, the disclosure o...
example 2
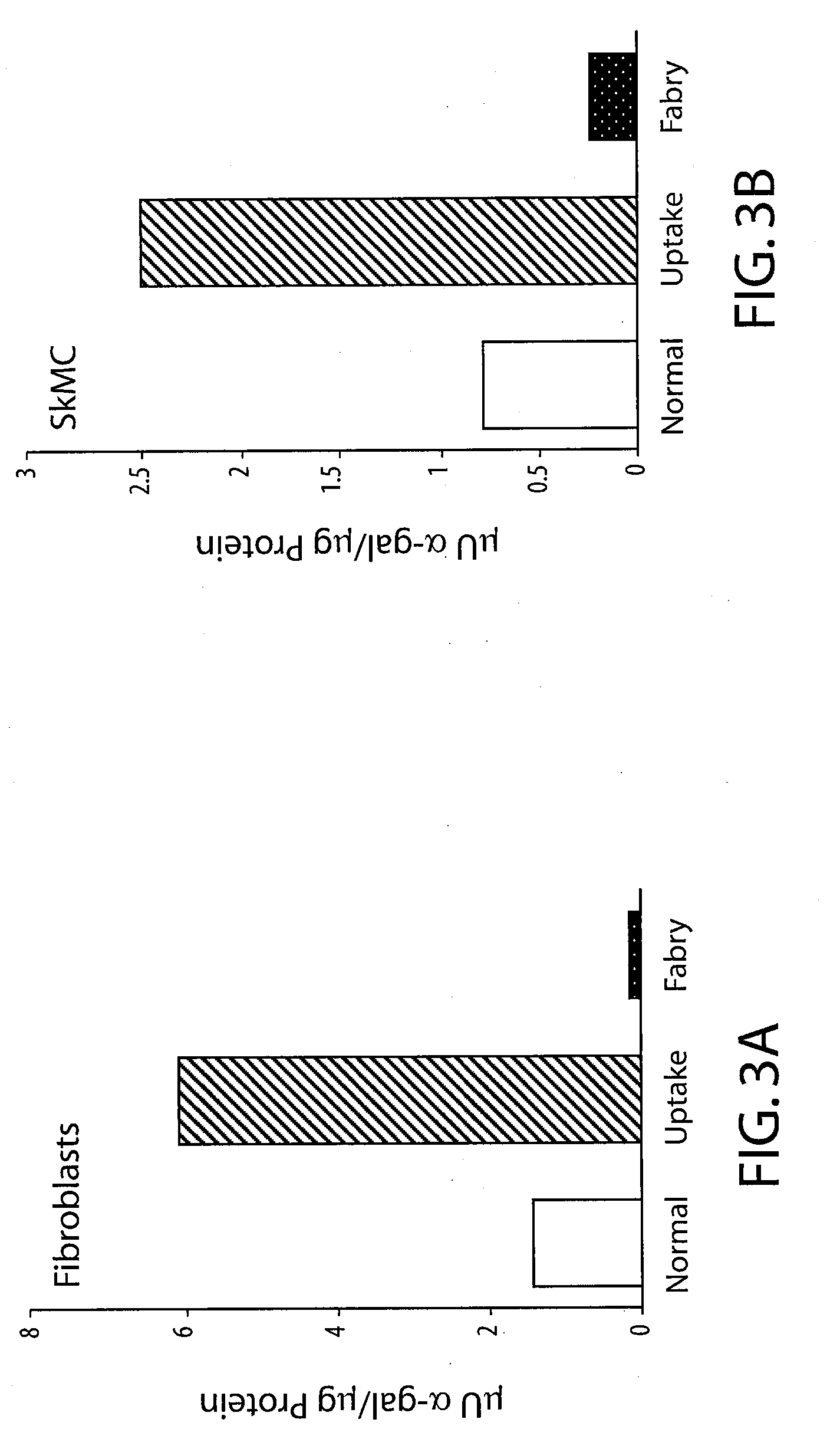
Uptake of Human α-galactosidase A Produced from Ad2 / CEHα-gal by Fabry Fibroblasts
[0068]Human primary cells were infected with Ad2 / CEHα-gal at the following MOIs (Fabry fibroblast cell line GM02775: 0, 2, 4, 6 and 8 μU α-gal / μg protein; skeletal muscle cell line SkMC: 0, 0.5, 1, 1.5, 2, 2.5 and 3 μU α-gal / μg protein). Three days after infection conditioned culture medium was collected and filtered to remove virus particles. Filtered conditioned medium was applied to uninfected Fabry fibroblasts (GM02775). After a five hour incubation, medium was removed, cells were washed with PBS, and harvested in 0.5 ml lysis buffer. Fibroblasts from normal (GM02770B) and Fabry donors which had not been exposed to conditioned medium were harvested and assayed as controls. Cell lysates were assayed using the fluorescent substrate 4-methylumbelliferyl-α-D-galactopyranoside (4-mu-α-gal). (FIGS. 3A and 3B). The assays showed that human primary cells infected with Ad2 / CEHα-gal secreted biologically acti...
example 3
Tissue Distribution of α-galactosidase A in Normal Vs. Fabry's Knockout Mice
[0069]Normal (C57BL / 6n) and Fabry knockout mice (provided by Dr. Robert Desnick, Mount Sinai School of Medicine, New York, N.Y.) were assayed for levels of α-galactosidase A using the 4-mu-α-gal activity assay. A full body perfusion was performed at the time of sacrifice and the organs were harvested and stored at −80.degree. C. Tissues were homogenized in assay buffer and put through several freeze-thaw cycles. Fabry mice showed significantly reduced levels of α-galactosidase A activity when compared to normal mice in all organs tested. (FIG. 4).
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com