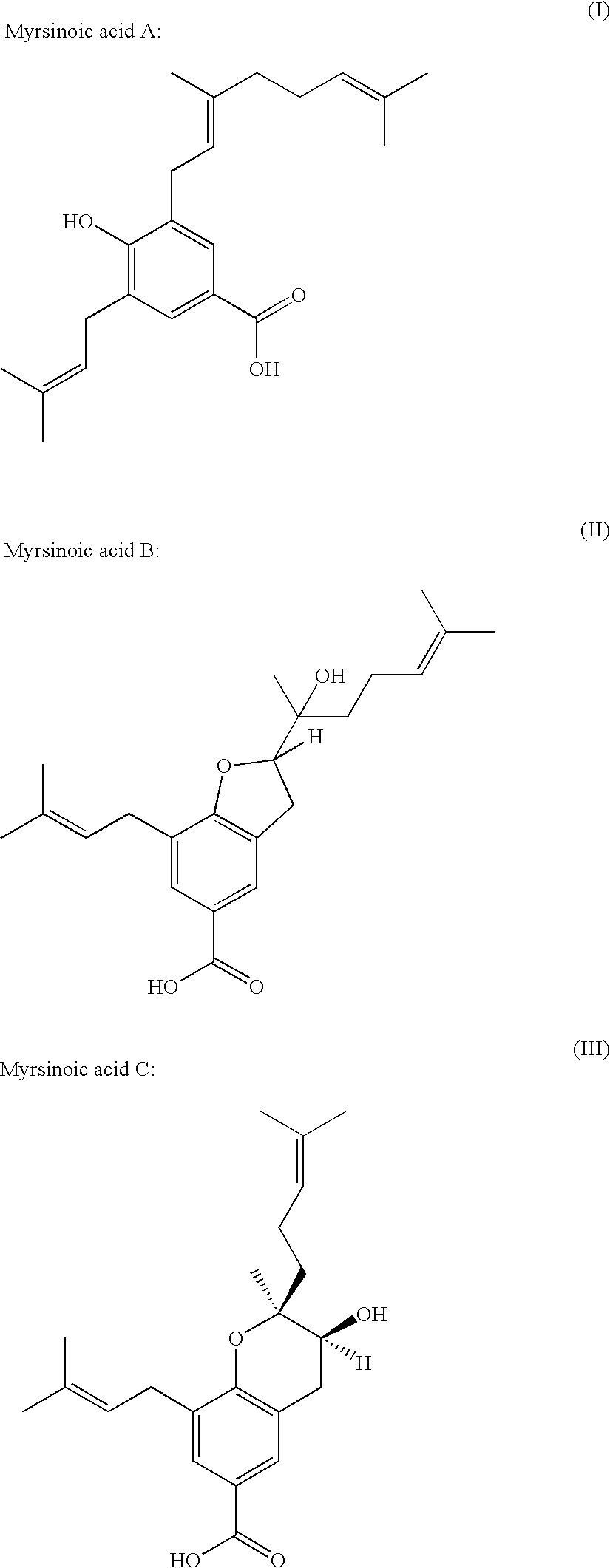
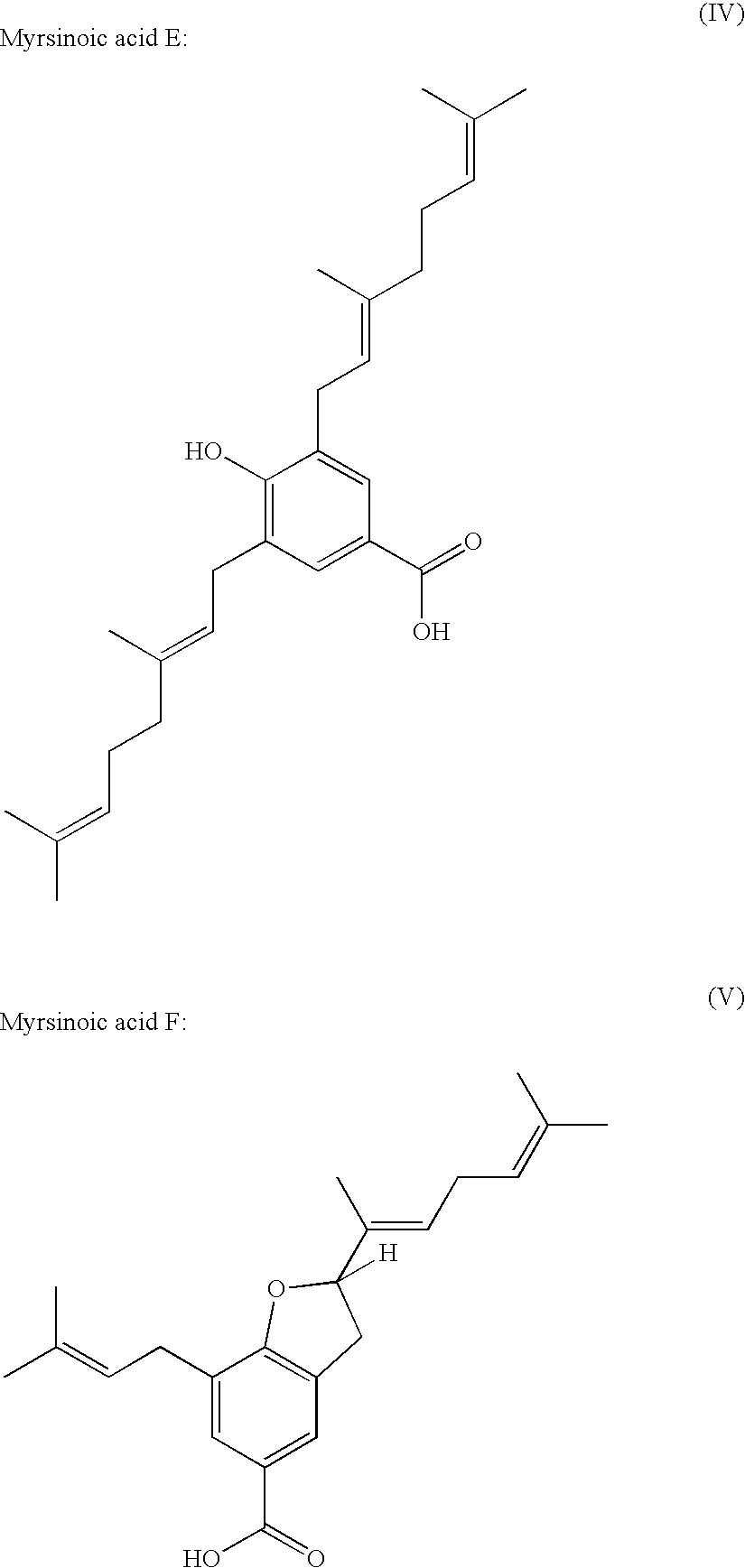
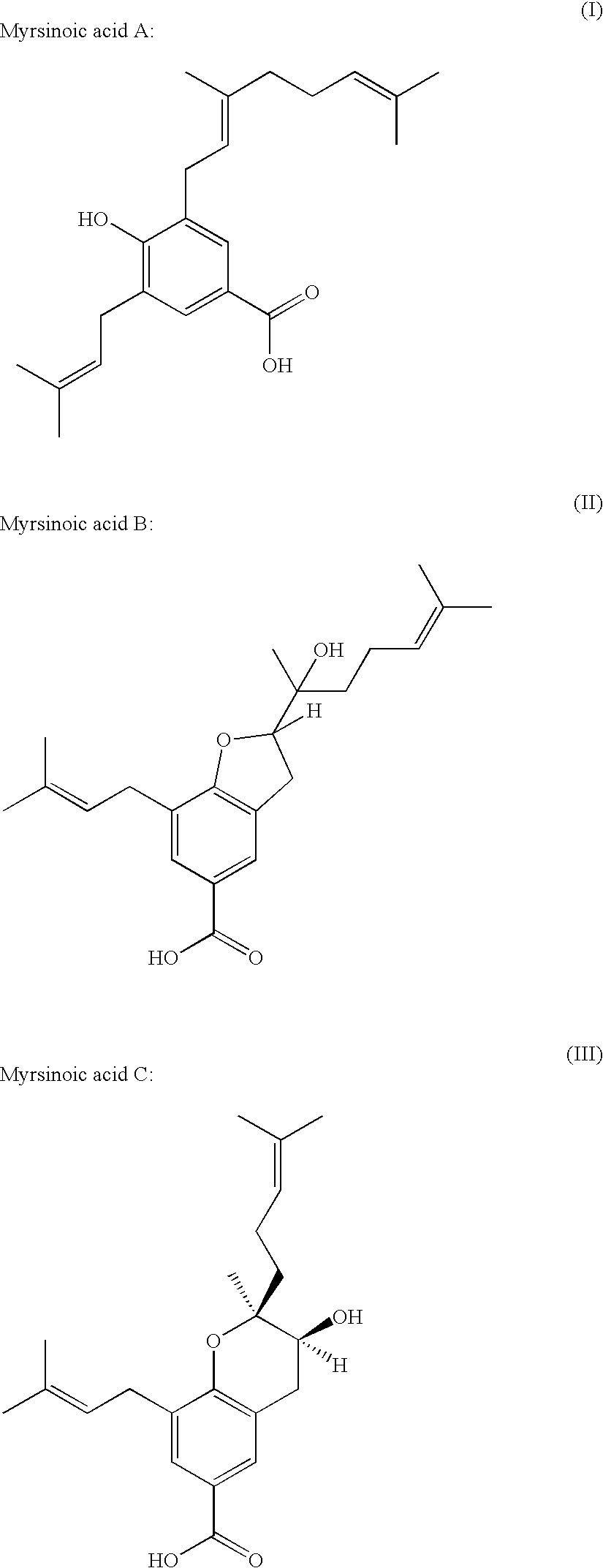
Methioninase inhibitor and composition and food or drink containing the same
a technology of methionine and composition, which is applied in the direction of drug composition, biocide, plant/algae/fungi/lichens ingredients, etc., can solve the problems of increased permeability of intraoral mucosa, growth and division of endothelial cells, and adverse effects of volatile sulfur compounds such as methyl mercaptan, so as to reduce the production of methyl mercaptan, reduce bad breath, and reduce feces odor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
(1) Sample Preparation Example 1
Preparation of Extracts by Water
[0039]To 5 g of a dry powder leaf of Myrsine seguinii, 50 mL of water was added for extraction at 70° C. for 2 hours. The obtained extract liquid was filtered and freeze-dried to obtain 0.75 g of an extract.
[0040]Similarly a twig and a fruit of Myrsine seguinii were extracted respectively by water, and the extract liquids were concentrated or freeze-dried to prepare the extracts. The yields of the extracts are shown in Table 1.
preparation example 2
(2) Sample Preparation Example 2
Preparation of Extracts by 25% Ethanol
[0041]To 5 g of a dry powder twig of Myrsine seguinii, 50 mL of 25% ethanol was added for extraction at 70° C. for 2 hours. The obtained extract liquid was filtered, the solvent was removed, and the residue was freeze-dried to obtain 0.80 g of an extract.
[0042]Similarly a leaf and a fruit of Myrsine seguinii were extracted respectively by 25% ethanol, and the extract liquids were concentrated or freeze-dried to prepare the extracts. The yields of the extracts are shown in Table 1.
preparation example 3
(3) Sample Preparation Example 3
Preparation of Extracts by 50% Ethanol
[0043]To 5 g of a dry powder fruit of Myrsine seguinii, 50 mL of 50% ethanol was added for extraction at 70° C. for 2 hours. The obtained extract liquid was filtered, the solvent was removed, and the residue was freeze-dried to obtain 0.80 g of an extract.
[0044]Similarly a leaf and a twig of Myrsine seguinii were extracted respectively by 50% ethanol, and the extract liquids were concentrated or freeze-dried to prepare the extracts. The yields of the extracts are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com