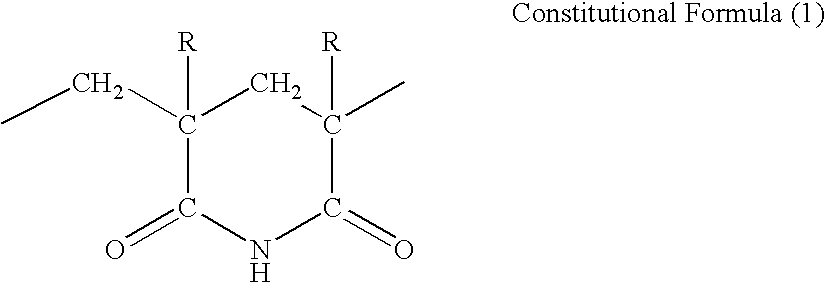
(METH)acrylic resin composition, imidized (METH)acrylic resin composition, and film obtained by molding them
a technology of acrylic resin and composition, which is applied in the field of (meth)acrylic resin composition, imidized (meth)acrylic resin composition, and film obtained by molding, can solve the problems of poor folding resistance of the film, poor chemical resistance of the conventional acrylic film, and poor resistance to aqueous alkali solution and the like, so as to improve the moldability, improve the alkali resistance, and improve the chemical resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1-1
Production of (Meth)Acrylic-Based Resin (C)
[0165]The following substances were loaded into an agitator-equipped 8 L polymerizer.
Deionized water200partsDioctyl sodium sulfosuccinate0.25partsSodium formaldehyde sulphoxylate0.15partsEthylenediaminetetraacetic acid disodium0.005partsFerrous sulfate0.0015parts
[0166]After the internal portion of the polymerizer was purified sufficiently with nitrogen gas so that oxygen was not substantially present, the internal temperature was set to 60° C., and 20 parts of the monomer mixture functioning as the starting material of the acrylic acid ester-based crosslinked elastic particle (B) shown in C1-1 in Table 1 (i.e., a monomer mixture comprising 2.1 parts of AlMA and 0.2 parts of CHP with respect to 100 parts of a monomer mixture comprising 90% of BA and 10% of MMA) were added continuously at a rate of 10 parts / hour. After the addition, the polymerization was allowed to continue for 0.5 hours, and an acrylic acid ester-based crosslinked elastic p...
example 4
[0174]With respect to 100 parts of the resin powder C1-4 of the (meth)acrylic-based resin (C), 50 parts of the methacrylic-based resin SUMIPEX LG (manufactured by Sumitomo Chemical Co., Ltd., acid value=0 mmol / g) were blended, and the mixture was melt-kneaded using a vented 40 mmφ single-screw extruder in which the cylinder temperature was set to 260° C., and resin pellets of the (meth)acrylic resin compositions (4) and (5) were obtained. Here, in the NMR measurement of the (meth)acrylic resin composition (4), the peak derived from a tertiary-butyl group near 1.3 to 1.5 ppm had disappeared, confirming that a desorption reaction had progressed.
[0175]The properties of the obtained (meth)acrylic resin composition were evaluated. The results together with the acid value of the (meth)acrylic resin composition are shown in Table 2.
[0176]It was confirmed by acid value measurement that a carboxylic acid group was present in the (meth)acrylic resin composition (4) obtained by blending the re...
example 5
[0177]With respect to 100 parts of the resin powder C1-5 of the (meth)acrylic-based resin (C), 50 parts of the methacrylic-based resin HT121 (manufactured by ALTUGLASS, acid value=0.45 mmol / g) were blended, and the mixture was melt-kneaded using a vented 40 mmφ single-screw extruder in which the cylinder temperature was set to 260° C., and resin pellets of the (meth)acrylic resin composition (5) were obtained. Here, in the NMR measurement of the (meth)acrylic resin composition (5), the peak derived from a tertiary-butyl group near 1.3 to 1.5 ppm had disappeared, confirming that a desorption reaction had progressed.
[0178]The properties of the obtained (meth)acrylic resin composition were evaluated. The results together with the acid value of the (meth)acrylic resin composition are shown in Table 2.
[0179]It was confirmed by acid value measurement that a carboxylic acid group was present in the (meth)acrylic resin composition (5) obtained by blending the resin powder C1-5 of the (meth)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt viscosity | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com