Nanostructured Metal Oxides Comprising Internal Voids and Methods of Use Thereof
a metal oxide and nanostructure technology, applied in the field of nanostructures, can solve the problems of poor cyclability, limiting the use of such applications, and the method is often burdened with the challenge of uniform depositing of metal oxides (or their precursors) on templates, and achieves the effects of less cost, improved cyclability, and improved cyclability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
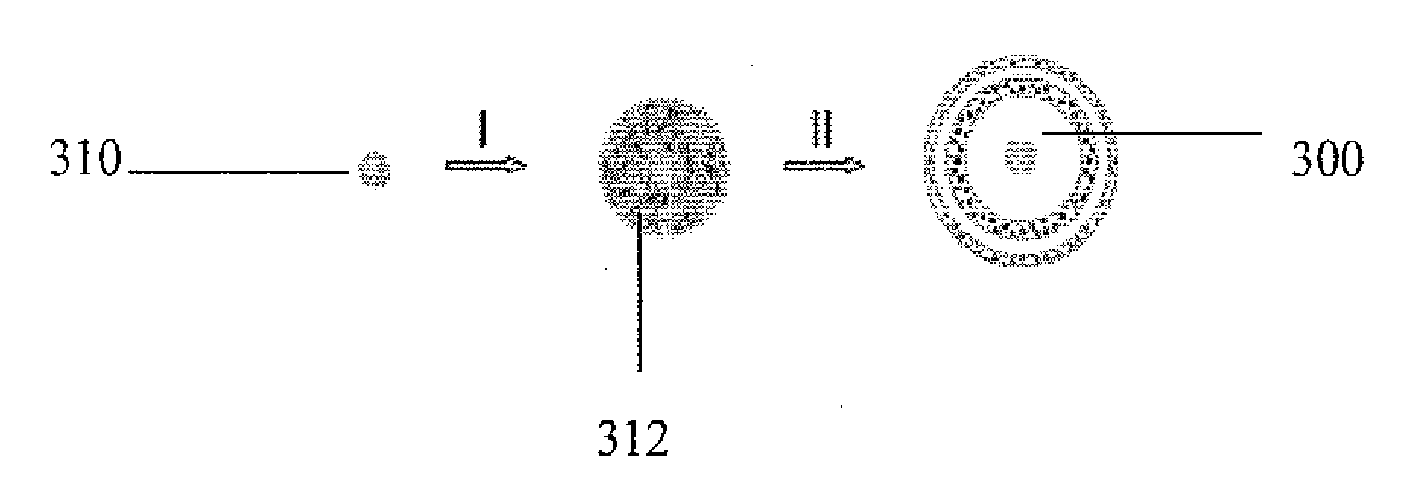
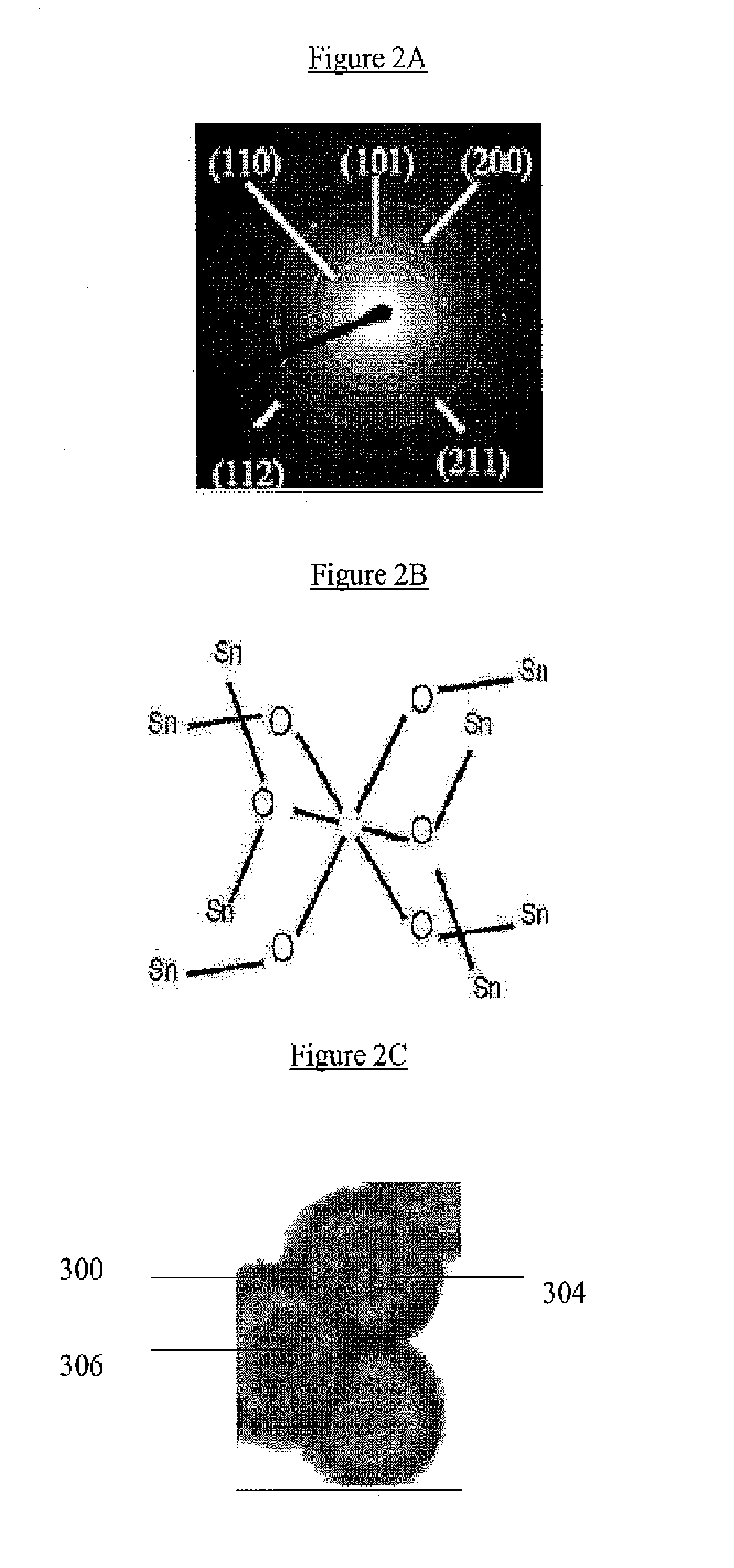
[0056]Hollow SnO2 nanoparticles were prepared by a hydrothermal method in an ethanol / H2O mixed solvent. Potassium stannate trihydrate (K2SnO3; 3H2O, Aldrich, 99.9%) was added to 30 ml of ethanol / H2O mixture with an r value of 25-50%, to achieve potassium stannate concentrations of 4.7 mM-40 mM. After gentle shaking by hand for about 5 minutes, a slightly white translucent or clear solution (depending on values of r and c) was obtained, which was then transferred to a 40 mL Teflon-lined stainless steel autoclave. In certain experiments, urea, thiourea or ethyl diamine were also used as additives, typically with overall concentrations of about 0.1 mM. After heating in an electric oven at 150° C. for a period of 3-48 hours, the autoclave was gradually cooled down in air, or rapidly using tap water. The white product was harvested by centrifugation and washed with deionized water and ethanol before drying at 50° C. overnight.
[0057]The electrochemical properties of the hollow SnO2 nanosp...
example 2
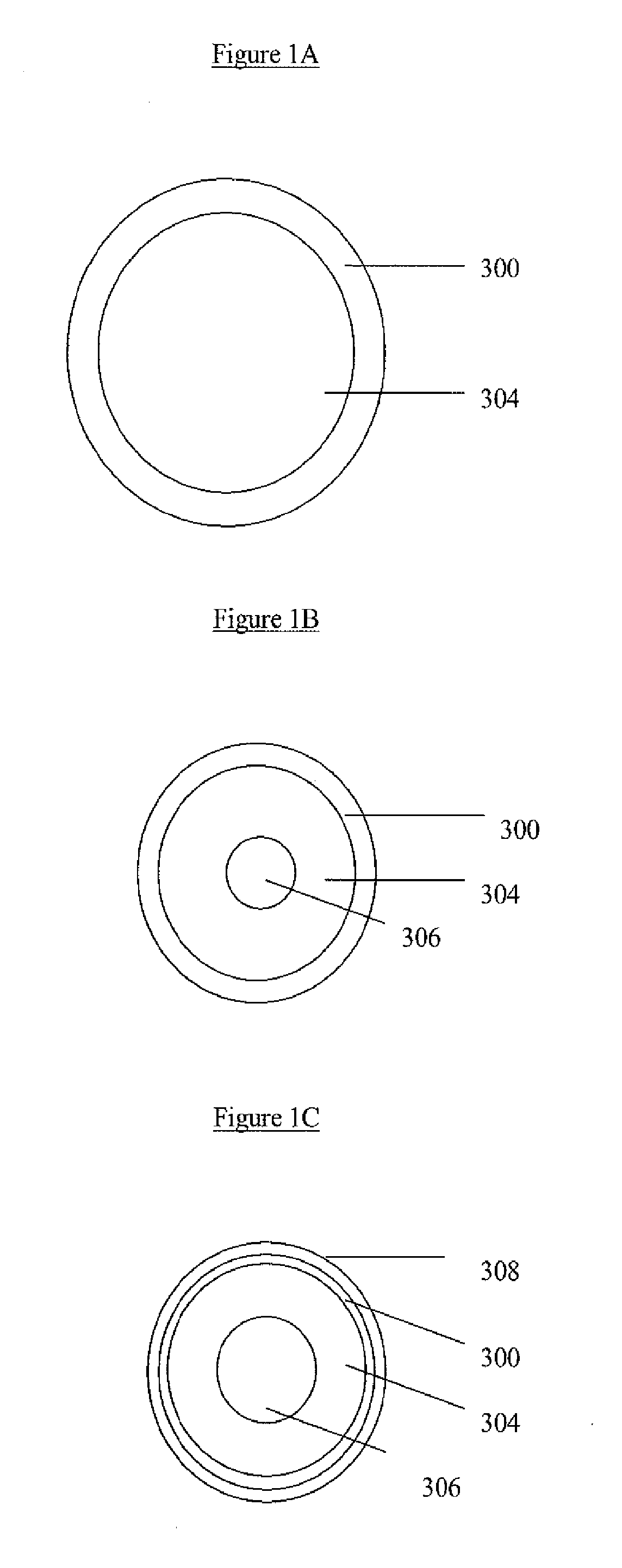
[0060]Monodisperse silica nanospheres with different sizes were prepared from the well known Stobers method. W. Stober, A. Fink, E. Bohn, J. Colloid Interface Sci. 1968, 26, 62-69. Polycrystalline SnO2 shells were facilely deposited on silica templates without any prior surface modification by a hydrothermal method. Depending on the amount of nanostructures desired, 40-120 mg silica nanospheres were first dispersed by ultrasonication in 30 ml of ethanol / water (37.5 vol % ethanol) mixed solvent. To this white suspension, urea (0.9 g or 0.5 M) and potassium stannate trihydrate (˜144 mg or 16 mM; K2SnO3.3H2O, Aldrich, 99.9%) were added. After shaking by hand for about 5 minutes until the salt dissolved, the suspension was transferred to a 40 ml Teflon-lined stainless-steel autoclave, which was then heated in an airflow electric oven at 150-190° C. for 36 hours. After the autoclave cooled down naturally, the white product was harvested by centrifugation and washed with deionized water. ...
example 3
[0061]The Au / silica core / shell particles were prepared as described in detail by Liu et al. S. H. Liu, M. Y. Han, Adv. Funct. Matter. 2005, 15, 961-967. After extensive washing with water, the as-obtained Au / silica core / shell particles (˜50 mg) were directly used without drying as the template for the same SnO2 deposition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com