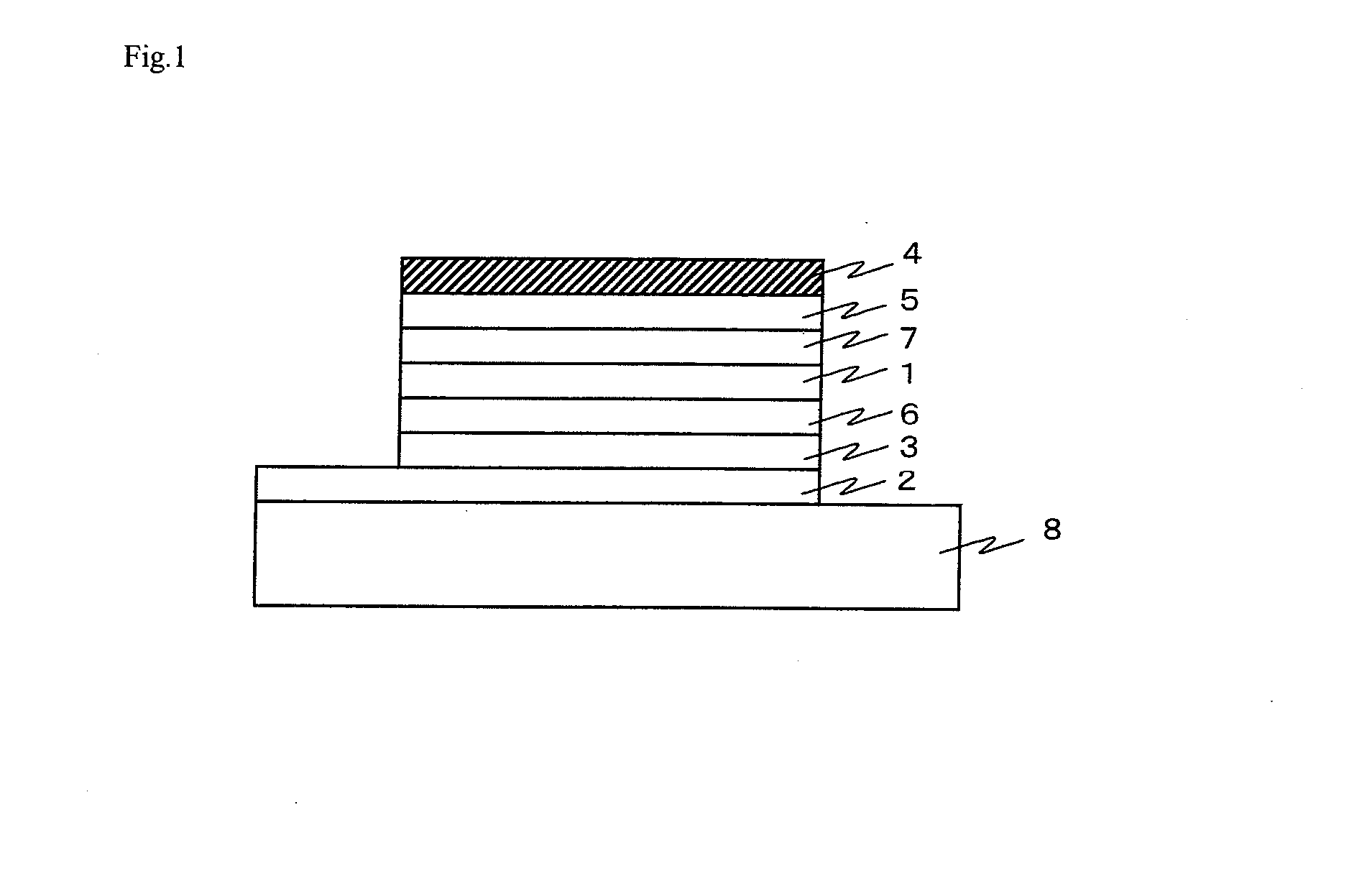
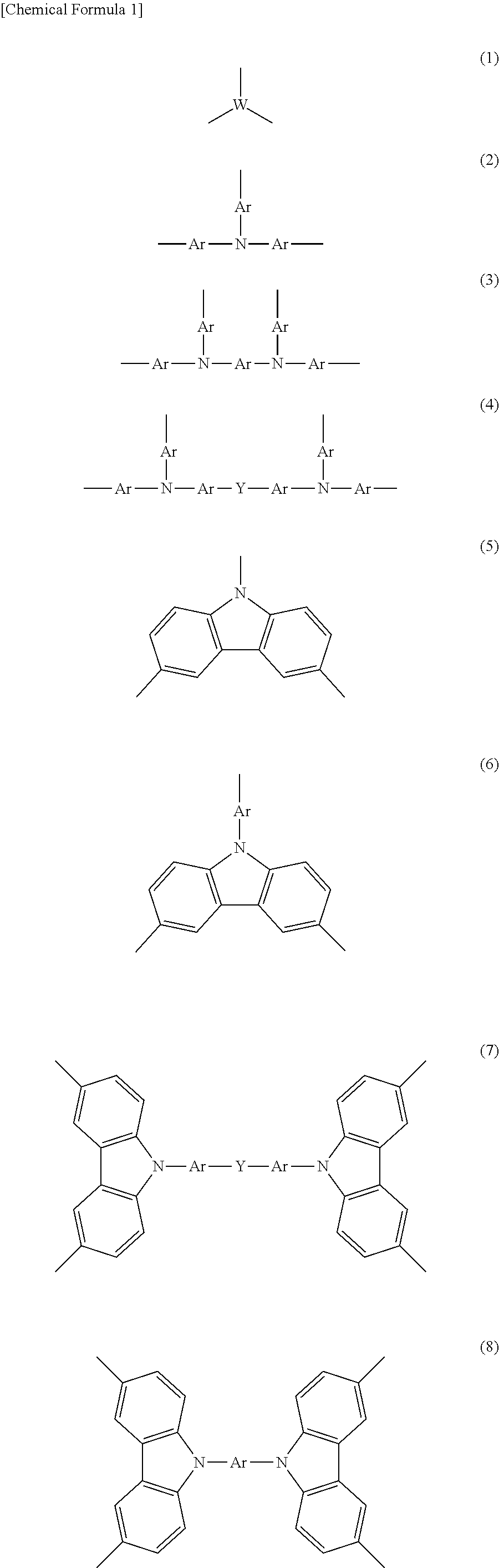
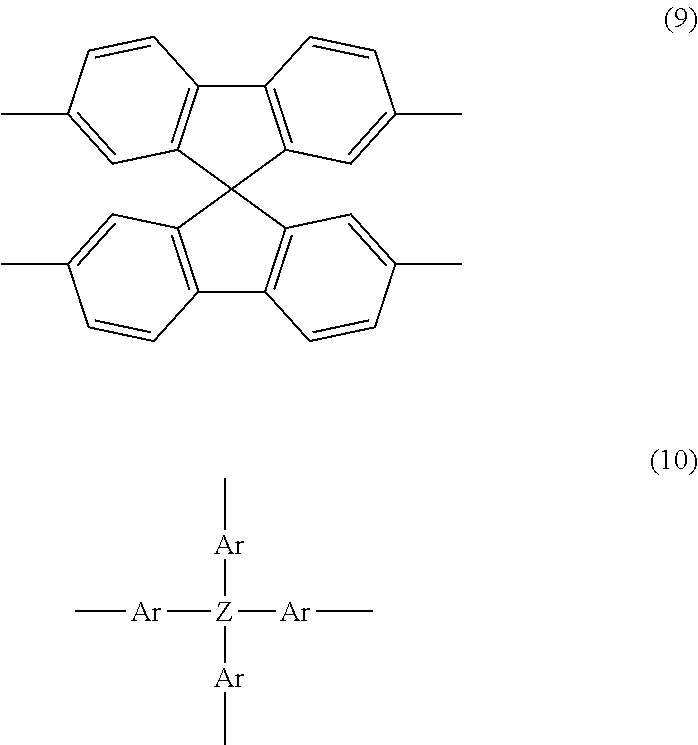
Organic Electronic Material, Ink Composition Containing Same, and Organic Thin Film, Organic Electronic Element, Organic Electroluminescent Element, Lighting Device, and Display Device Formed Therewith
a technology of organic electronic elements and ink compositions, which is applied in the direction of conductive materials, solid-state devices, inks, etc., can solve the problems of short element lifetime, low light emission efficiency and short element lifetime, and difficulty in providing a multi-layer structure in polymer-type organic el elements, etc., to achieve superior light emission efficiency, easy formation, and superior emission lifetime
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0297]A PEDOT:PSS dispersion liquid (manufactured by Starck-Vtech, Ltd., A14083 LVW142) was spin coated at 1500 min−1 on a glass substrate having an ITO pattern with a width of 1.6 mm, and the glass substrate was heated to dry on a hot plate at 200° C. for 10 minutes in air. Thus, a hole injection layer (40 nm) was formed. The experiment thereafter was carried out in a dry nitrogen environment.
[0298]Subsequently, a coating solution prepared by mixing the oligomer A (4.5 mg) obtained as described above, a photoinitiator (0.13 mg) which was the ionic compound 1, and toluene (1.2 mL), was spin coated at 3000 min−1 on the hole injection layer, and then the coating solution was irradiated with light (3 J / cm2) using a metal halide lamp. The coating solution was cured by heating on a hot plate at 180° C. for 60 minutes, and thus a hole transport layer (40 nm) was formed.
[0299]Subsequently, the glass substrate thus obtained was transferred into a vacuum deposition apparatus, and CBP+Ir(piq)...
example 2
[0304]
[0305]An element was produced in the same manner as in Example 1, except that a coating solution prepared by mixing the oligomer 13 (4.5 mg) obtained as described above, the initiator (the same as in Example 1) (0.45 mg), and toluene (1.2 mL) was spin coated at 3000 min−1 on the hole injection layer, the coating solution was cured by heating on a hot plate at 120° C. for 10 minutes, and thus a hole transport layer (40 nm) was formed. ITO of this organic EL element was used as the positive electrode, while Al was used as the negative electrode, and a voltage was applied. Red light emission was observed at 4.0 V, and the current efficiency at a luminance of 1000 cd / m2 was 5.0 cd / A. Further, the lifetime characteristics were measured, and the luminance decreased by half in 140 hours.
[0306]
example 3
[0307]A coating solution prepared by mixing the oligomer A (4.5 mg) obtained as described above, the photoinitiator (the same as in Example 1) (0.13 mg), and toluene (500 μL) was spin coated at 3000 min−1 on a glass substrate having an ITO pattern with a width of 1.6 mm. The operation thereafter was carried out in a dry nitrogen environment.
[0308]Subsequently, the coating solution was irradiated with light (3 J / cm2) using a metal halide lamp, and was cured by heating on a hot plate for 15 minutes at 120° C. and for 60 minutes at 180° C. Thus, a hole injection layer (40 nm) was formed.
[0309]Next, sealing was carried out in the same manner as in Example 1, while depositing CBP+Ir(piq)3 (40 nm), BAlq (10 nm), Alq3 (30 nm), LiF (film thickness 0.5 nm), and Al (film thickness 100 nm) in this order.
[0310]ITO of this organic EL element was used as the positive electrode, while Al was used as the negative electrode, and a voltage was applied to the element. Red light emission was observed a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com