Heteroaromatic-based electrolytes for lithium and lithium-ion batteries
a lithium-ion battery and electrolyte technology, applied in the field of electrolyte for lithium and lithium-ion batteries, to achieve the effects of stable over a wide temperature range, excellent ionic conductivity, and low cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
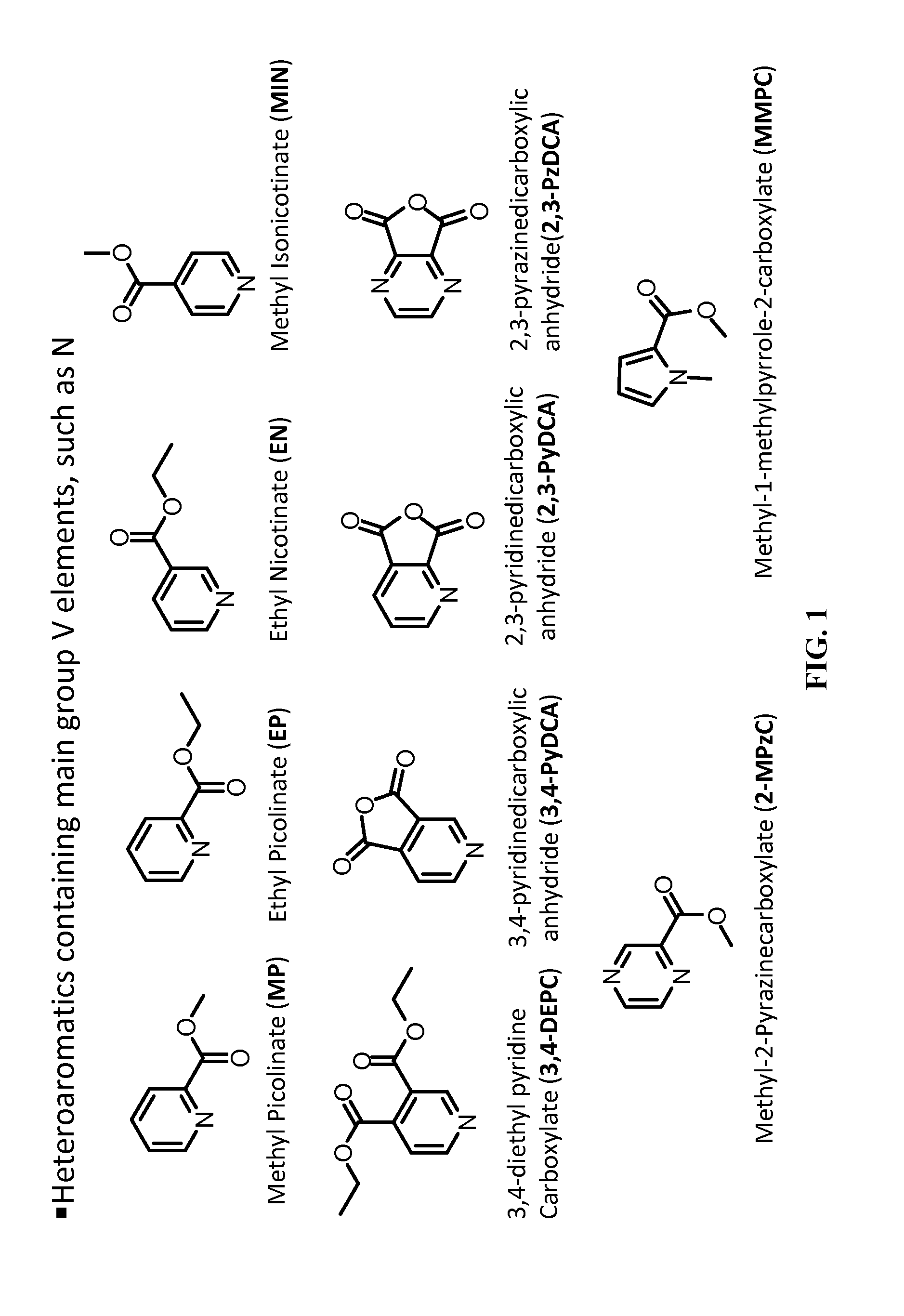
[0069]An electrolyte composition designated as Gen2 was prepared, comprising 1.2 M LiPF6 in a 3:7 (w / w) mixture of ethylene carbonate (EC) and ethyl methyl carbonate (EMC) Electrolytes of the invention were prepared by adding about 0.3 wt % of either methyl picolinate (MP) or ethyl picolinate (EP) to the Gen2 electrolyte. The electrolytes were then evaluated in an electrochemical cell including a cathode comprising a 35 micron thick coating of Li0.08Co0.15Al0.05O2 on an aluminum collector plate, an anode comprising a 35 micron thick coating of 5 micron graphite particles on a copper collector plate, and a 25 micron CELGARD® 3501 separator membrane.
[0070]FIG. 6 is a plot of discharge capacity versus cycle number for electrochemical cells at 30° C. over a 3 to 4.1V range for the electrolytes containing 0.3 wt % of MP or EP compared to the Gen2 control. The 1st and seconds cycles were run at a C / 12 rate, and the next 50 cycles were run at a C / 4 rate. The results in FIG. 6 demonstrate t...
example 2
[0072]Electrolytes containing about 0.3 wt % of either MP, EP, methyl isonicotinate (MIN), or ethyl nicotinate (EN) added to the Gen2 electrolyte of Example 1 were evaluated in a cell of the same design as described in Example 1. FIG. 8 is a plot of discharge capacity versus cycle number for the electrochemical cells, which shows that addition of 0.3 wt % of the heteroaromatic additives to the Gen2 electrolyte improved the retention capacity relative to the Gen2 electrolyte. MIN provided the least initial capacity loss.
[0073]FIG. 9 is a plot of dQ / dV over a voltage range of about 1.8 to about 4.2 volts the cells, which shows that addition of 0.3 wt % of the heteroaromatic compounds to the Gen2 electrolyte induced significant changes between 1.8 V and 3V, indicating that reactions with graphite occurred.
[0074]FIG. 10 is a plot of AC impedance data for the electrochemical cells, which shows that addition of 0.3 wt % of the heteroaromatic compounds to the Gen 2 electrolyte did not sign...
example 3
[0075]Electrolytes containing about 0.3 wt % of MP added to the Gen2 electrolyte of Example 1 were evaluated in a cell of the same design as described in Example 1 with the formation cycle being run at about 30° C. or 55° C. FIG. 11 is a plot of discharge capacity versus cycle number for the electrochemical cells compared to the control Gen2 electrolyte. The results in FIG. 11 indicate that the initial capacity of the cell that included MP in the electrolyte increased when the formation cycle was performed at the higher temperature. Capacity improvement is indicative of improved electrode “wetting” by the electrolyte.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com