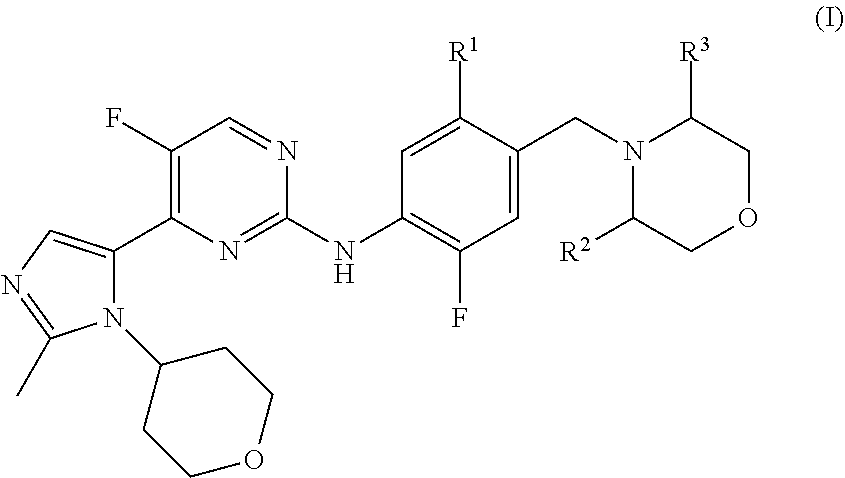
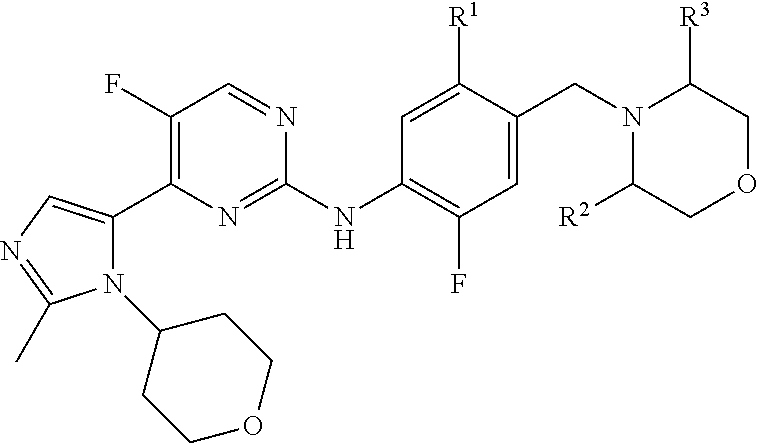
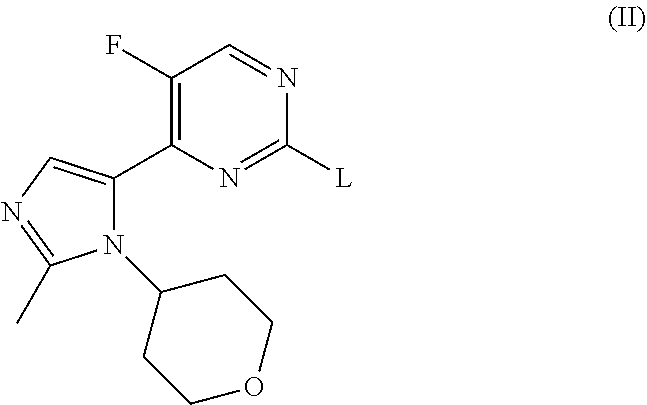
Imidazole Substituted Pyrimidines Useful in the Treatment of Glycogen Synthase Kinase-3 Related Disorders such as Alzheimer's Disease
a glycogen synthase and kinase-related technology, applied in the field ofimidazole substituted pyrimidines useful in the treatment of glycogen synthase-related disorders such as alzheimer's disease, can solve the problems of lithium intoxication, axon and neuritic dystrophy, etc., and achieve the effect of good bioavailability and selective inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working examples
[0145]The following working example will describe, but not limit, the invention.
example 1
5-Fluoro-N-(2-fluoro-4-(morpholinomethyl)phenyl)-4-(2-methyl-1-(tetrahydro-2H-pyran-4-yl)-1H-imidazol-5-yl)pyrimidin-2-amine
[0146]
[0147]5-Fluoro-4-(2-methyl-1-(tetrahydro-2H-pyran-4-yl)-1H-imidazol-5-yl)pyrimidin-2-amine (5.12 g, 18.46 mmol), 4-(4-bromo-3-fluorobenzyl)morpholine (5.06 g, 18.46 mmol), (1,1′-bis(diphenylphosphino)ferrocene)-dichloropalladium(II) (0.152 g, 0.18 mmol), 9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene (0.107 g, 0.18 mmol) and sodium tert-pentoxide (4.07 g, 36.92 mmol) in toluene (70 mL) were mixed and degased. The mixture was stirred at 110° C. under nitrogen atmosphere overnight. The mixture was concentrated. Dichloromethane and water were added and the phases were separated. The organic phase was concentrated and the crude was purified by preparative HPLC. The pooled fractions were concentrated to about half volume. The residue was extracted with EtOAc (×2). The combined organic phases were concentrated and the residue was dried in vacuum at 40° C. for ...
example 2
5-Fluoro-N-(2-fluoro-4-(((R)-3-methylmorpholino)methyl)phenyl)-4-(2-methyl-1-(tetrahydro-2H-pyran-4-yl)-1H-imidazol-5-yl)pyrimidin-2-amine hydrochloride
[0153]
[0154](R)-4-(4-Bromo-3-fluorobenzyl)-3-methylmorpholine (200 mg, 0.69 mmol), 5-fluoro-4-(2-methyl-1-(tetrahydro-2H-pyran-4-yl)-1H-imidazol-5-yl)pyrimidin-2-amine (192 mg, 0.69 mmol) and potassium tert-butoxide (78 mg, 0.69 mmol) were mixed in dioxane (4 ml) and the mixture was stirred under a stream of argon for 5 minutes. Pd2(dba)3 (76 mg, 0.08 mmol) and X-Phos (79 mg, 0.17 mmol) were added followed by DMF (1 ml) and the reaction mixture was heated in a microwave reactor at 120° C. for 40 minutes. The crude was filtered through diatomeous earth. The filtrate was diluted with dichloromethane and was washed with brine. The aquous phase was extracted with dichloromethane (×3). The organic phases were pooled, evaporated and purified by column chromatography on silica followed by preparative HPLC. Fractions containing product were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| capillary voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com