Borrelia antigens
a technology of borrelia and antigens, applied in the field of isolated nucleic acid molecules, can solve the problems of withdrawn from the market, high cost of preventing the disease, autoimmune reactions, etc., and achieve the greatest potential of preventing lyme borreliosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Screening Procedure for the Identification of the Peptides According to the Present Invention
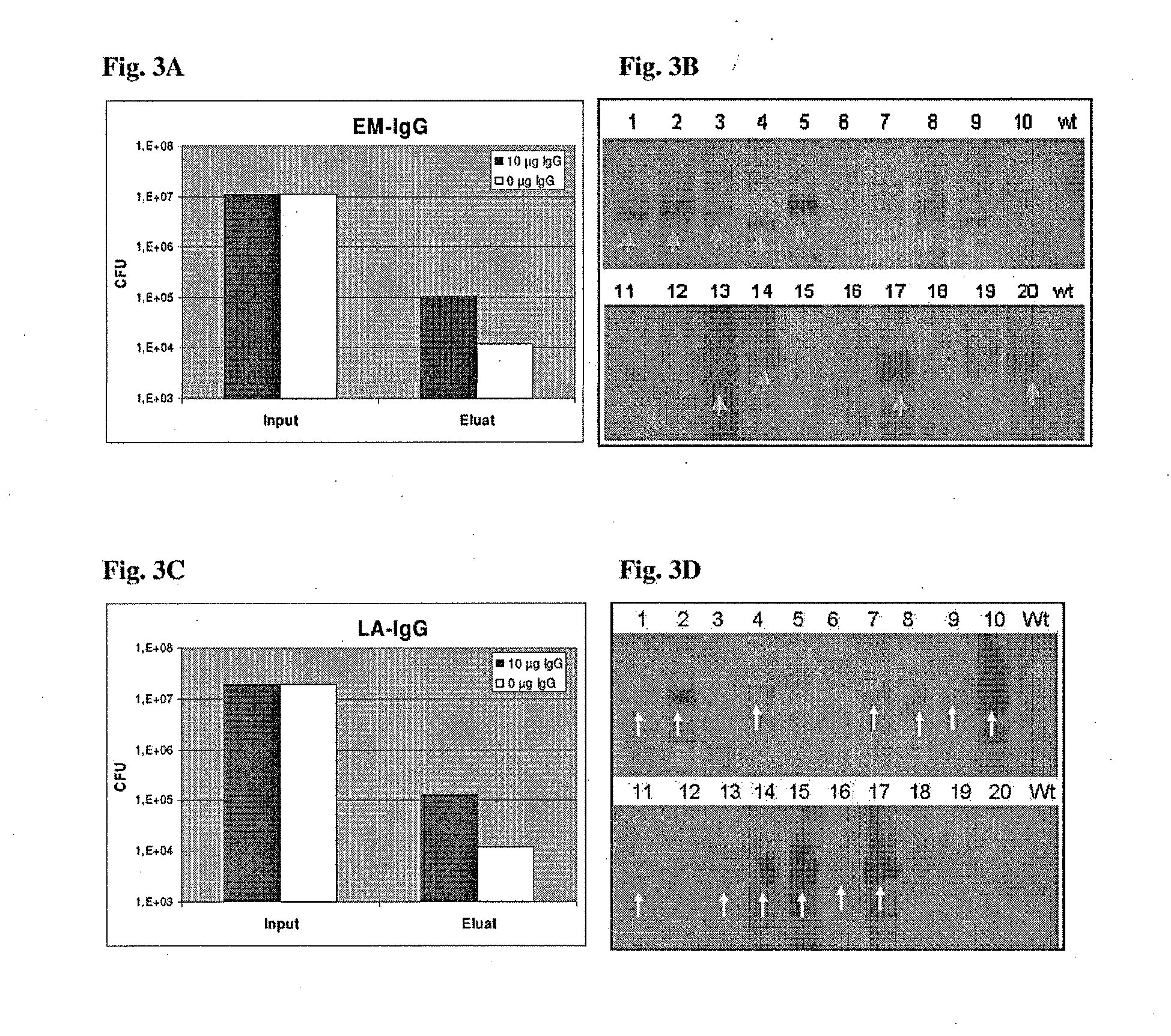
[0296]The approach, which has been employed for the present invention, is based on the interaction of proteins or peptides encoded by B. burgdorferi s.l. with the antibodies present in human sera. The antibodies produced against B. burgdorferi s.l. by the human immune system and present in human sera are indicative of the in vivo expression of the antigenic proteins and to their immunogenicity. In addition, the antigenic proteins as identified by the bacterial surface display expression libraries using pools of pre-selected sera, are processed in a second and third round of screening by individual selected or generated sera. Thus the present invention supplies an efficient, relevant, comprehensive set of antigens as a pharmaceutical composition, especially a vaccine preventing infections caused by B. burgdorferi s.l.
[0297]In the antigen identification program for identifying a compre...
example 2
Characterization and Selection of Human Serum Sources Based on Anti-B. burgdorferi s.l. Antibodies, Preparation of Antibody Screening Reagents
Experimental Procedures
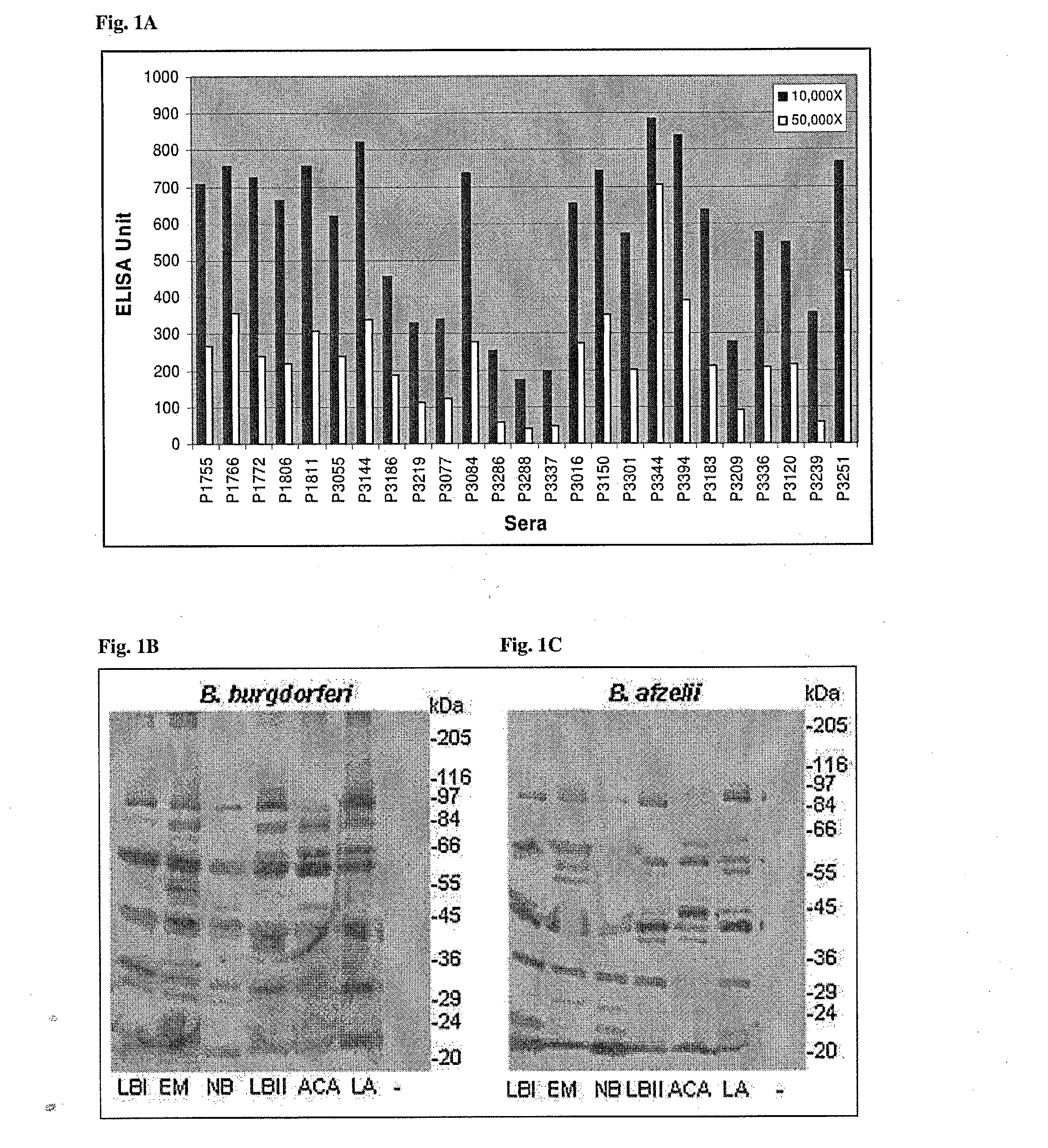
Enzyme-Linked Immunosorbent Assay (ELISA).
[0309]ELISA plates (Maxisorb, Millipore) were coated with 5-10 μg / ml total protein diluted in coating buffer (0.1 M sodium carbonate pH 9.2). For whole cell ELISA, 106 biotin-labelled and fixed bacteria were added to Streptavidin-coated ELISA plates. Two dilutions of sera (10,000×, 50,000×) were made in PBS-BSA. Highly specific Horse Radish Peroxidase (HRP)-conjugated anti-human IgG secondary antibodies (Southern Biotech) were used according to the manufacturer's recommendations (dilution: 1,000×). Antigen-antibody complexes were quantified by measuring the conversion of the substrate (ABTS) to coloured product based on OD405 nm readings by automatic ELISA reader (TECAN SUNRISE).
Preparation of Bacterial Antigen Extracts.
[0310]Total bacterial lysate: The B. burgdorferi s.s. strain...
example 3
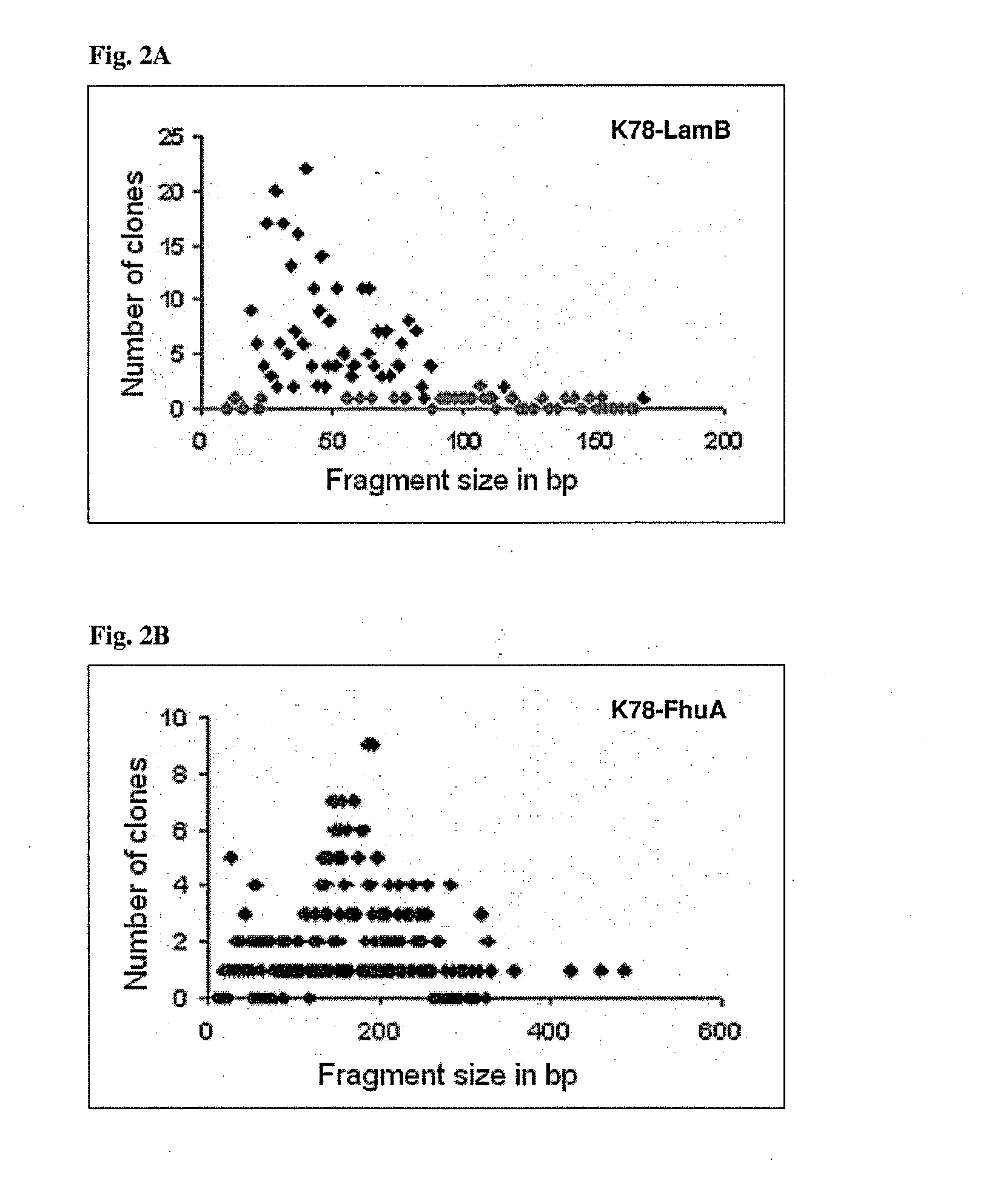
Generation of Highly Random, Frame-Selected, Small-Fragment, Genomic DNA Libraries of B. burgdorferi s.l.
Experimental Procedures
Preparation of Genomic DNA.
[0316]Cells from a 400 ml bacterial culture were harvested (5,000 rpm, 20 min, room temperature), washed with 80 ml 50 mM Tris pH 7.4 and re-suspended in 10 ml 50 mM Tris pH 7.4 / 25% Sucrose / 50 mM EDTA. The suspension was transferred to a fresh glass tube and Lysozyme (final conc.: 1.5 mg / ml) and SDS (final conc.: 2%) were added. The tube was incubated on ice for cell lysis. Proteinase K (final conc.: 0.1 mg / ml) was added and incubated for 10 min at 37° C., followed by Phenol / Chloroform (1:1) extraction, which was performed several times. A final extraction step was performed with Chloroform / Isoamylalcohol (1:24) to remove Phenol traces. The sample was treated with RNase A (final conc.: 10 μg / ml) for 1 h at room temperature and Phenol / Chloroform and Chloroform / Isoamylalcohol extractions were performed as described above. DNA in the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| immunogenic composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com