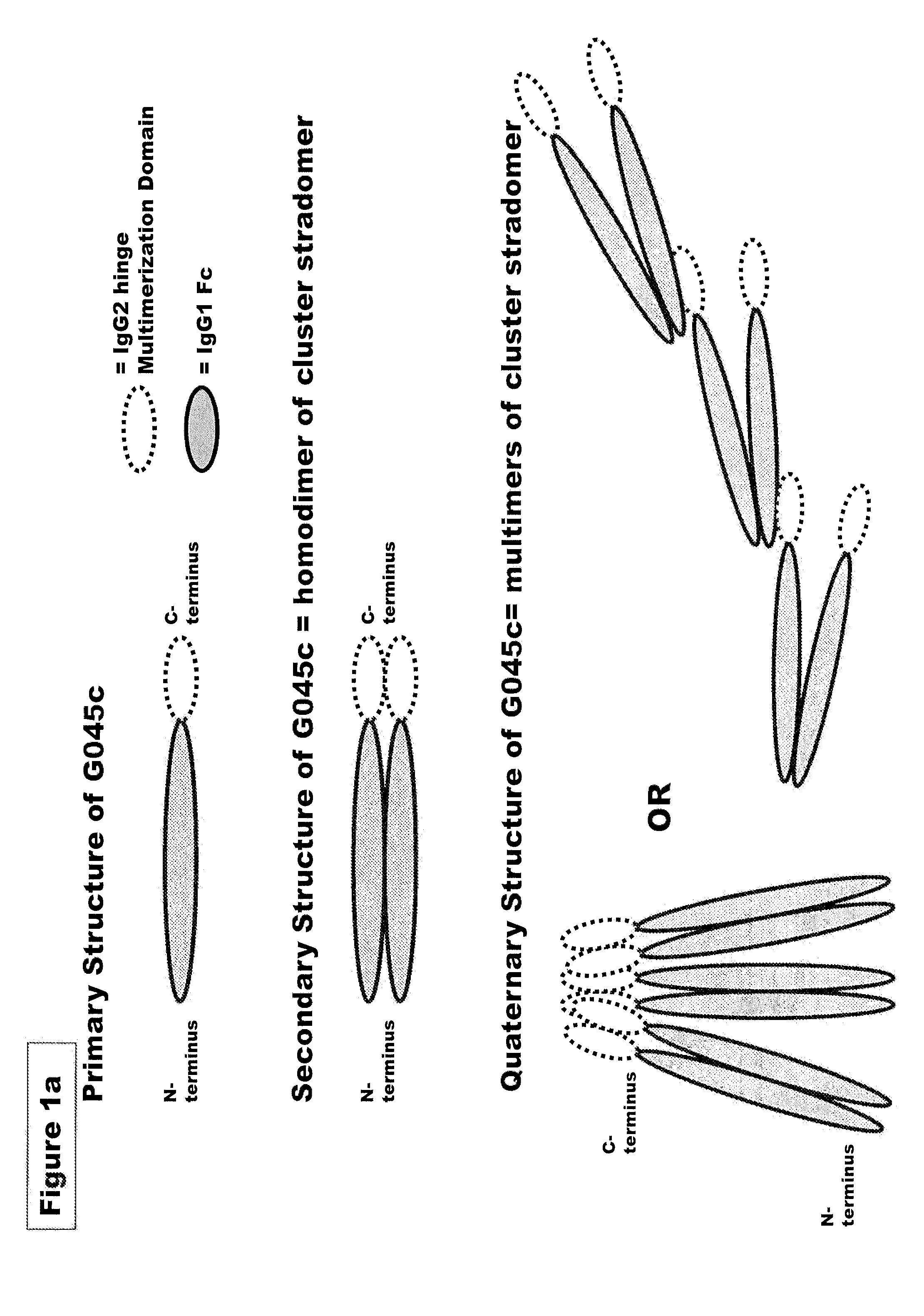
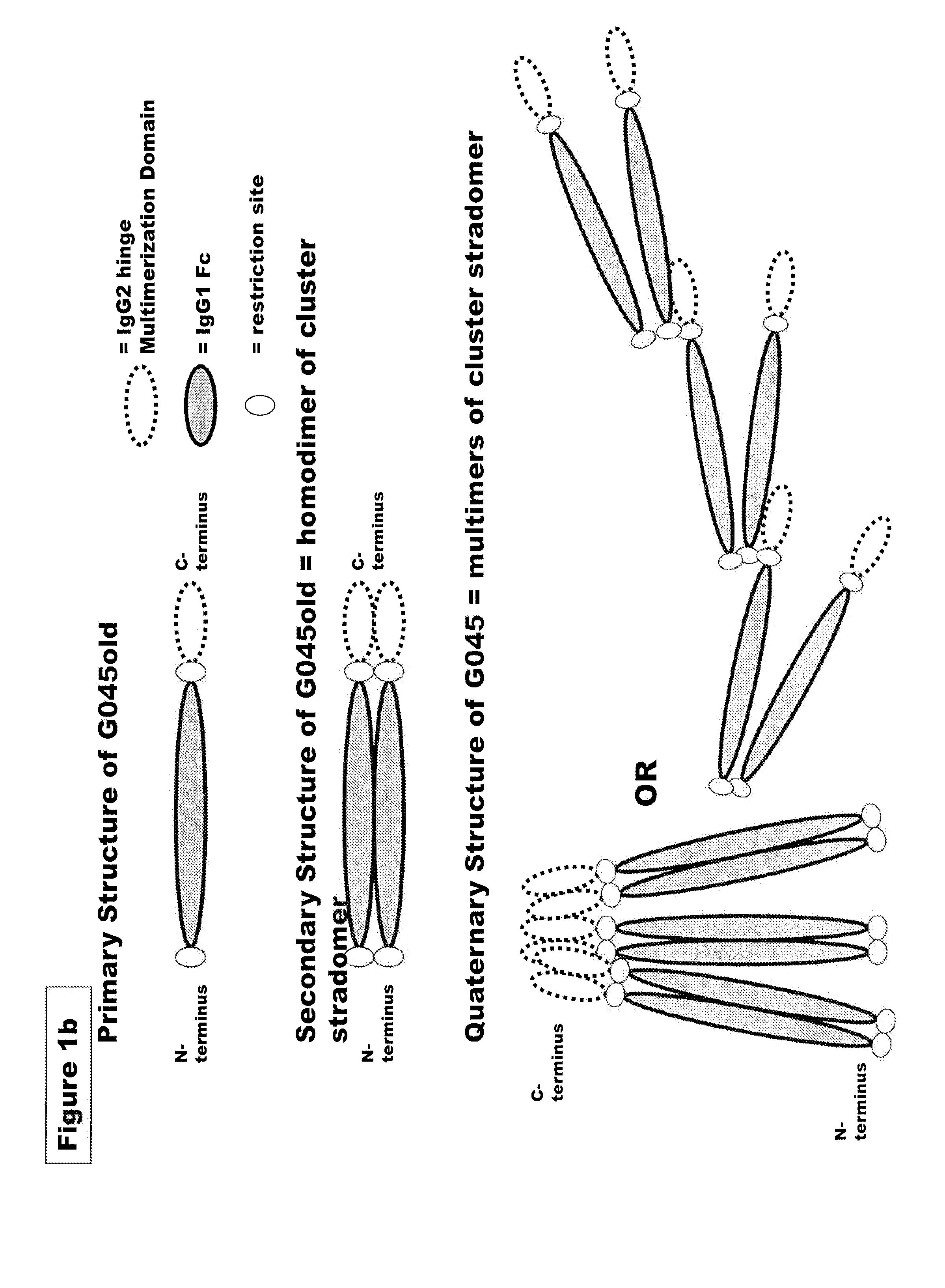
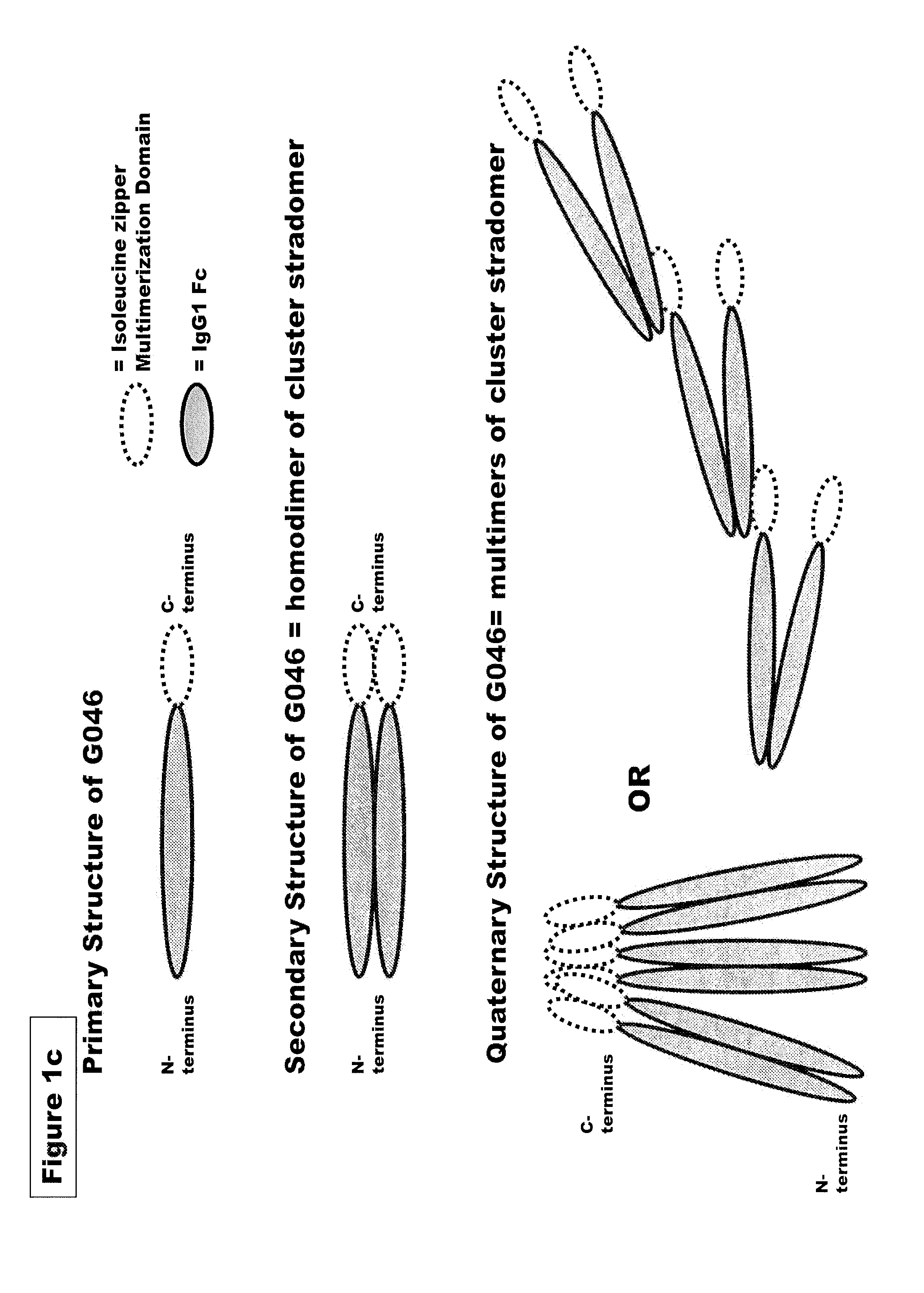
FUSION PROTEINS OF NATURAL HUMAN PROTEIN FRAGMENTS TO CREATE ORDERLY MULTIMERIZED IMMUNOGLOBULIN Fc COMPOSITIONS
a technology of immunoglobulin and fusion proteins, which is applied in the field of immunology, autoimmunity, inflammation, tumor immunology, etc., can solve the problems of insufficient sterility, lack of availability of pooled human blood products, and high cost, and achieves broad application, high protein load, and enhanced ivig efficacy. effect of aggregate fraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production and Purification of the Stradomers
[0227]HEK293F cells (Invitrogen, Carlsbad, Calif.) or Chinese Hamster Ovary Cells (CHO) were used for stable expression of G045 / M045 and G051. The HEK293F or CHO cells were grown in suspension for scale up of protein expression.
[0228]Genes encoding G045c (SEQ ID NO:4), G045old (SEQ ID NO:7) G051 (SEQ ID NO:18), G019 (SEQ ID NO: 8), G028 (SEQ ID NO:9), G046 (SEQ ID NO: 10), G075 (SEQ ID NO: 20), G076 (SEQ ID NO: 21), G096 (SEQ ID NO: 28), G098 (SEQ ID NO: 24), G089 (SEQ ID NO: 27), or the corresponding murine sequences of the preceding stradomers. were cloned into a vector containing a neomycin resistance gene such as pcDNA3.3 from Invitrogen (Carlsbad, Calif.) and under the transcriptional control of the CMV promoter to facilitate high level expression of G045 or G051. Plasmid DNA for transfection were isolated from bacterial culture using a endotoxin free plasmid DNA isolation kit (Nucleobond, Macherey-Nagel). G045c / G045old and G051 enco...
example 2
Enhanced Efficacy of M045c Compared with M045old in a Mouse Model of Arthritis
[0234]Assessment of the efficacy of M045c compared to that of M045old in collagen induced arthritis was performed. At day 0 and day 21 DBA1 / J mice were immunized with Type II bovine collagen (Chondrex, Inc., Cat. 20021) with a 4 mg / ml solution emulsified with Incomplete Freund's Adjuvant (Sigma, Cat #5506). The mice were weighed weekly and scored daily for signs of arthritis. Each paw was scored and the sum of all four scores was recorded as the Arthritic Index (AI). The maximum possible AI was 16 as follows: 0=no visible effects of arthritis 1=edema and / or erythema of one digit 2=edema and / or erythema of 2 joints 3=edema and / or erythema of more than 2 joints 4=severe arthritis of the entire paw and digits including limb deformation and ankylosis of the joint. Starting at day 22 (treatment day 0) ten of the collagen immunized mice were sorted into treatment groups based upon average AI (3.3) and ten non-di...
example 3
Synergistic Effect of M045 and Prednisolone in a Mouse Model of Arthritis
[0237]Assessment of efficacy of M045c combined with low dose prednisolone in a collagen induced arthritis model was performed. Briefly at day 0 and day 21 DBA1 / J mice were immunized with Type II bovine collagen (Chondrex, Inc., Cat. 20021) with a 4 mg / ml solution emulsified with Incomplete Freund's Adjuvant (Sigma, Cat #5506). The mice were weighed weekly and scored daily for signs of arthritis. Each paw was scored and the sum of all four scores was recorded as the Arthritic Index (AI). The maximum possible AI was 16 as follows: 0=no visible effects of arthritis 1=edema and / or erythema of one digit 2=edema and / or erythema of 2 joints 3=edema and / or erythema of more than 2 joints 4=severe arthritis of the entire paw and digits including limb deformation and ankylosis of the joint. Starting at day 22 (treatment day 0) ten of the collagen immunized mice were sorted into treatment groups based upon average AI (3.3)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com