Modulators of the prostacyclin (PGI2) receptor useful for the treatment of disorders related thereto
a prostacyclin and receptor technology, applied in the preparation of urea derivatives, cardiovascular disorders, drug compositions, etc., can solve the problems of unable to register for the treatment of pah in europe, complicated intravenous treatment, and patients at risk of potentially fatal rebound pulmonary hypertension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Syntheses of Compounds of the Present Invention
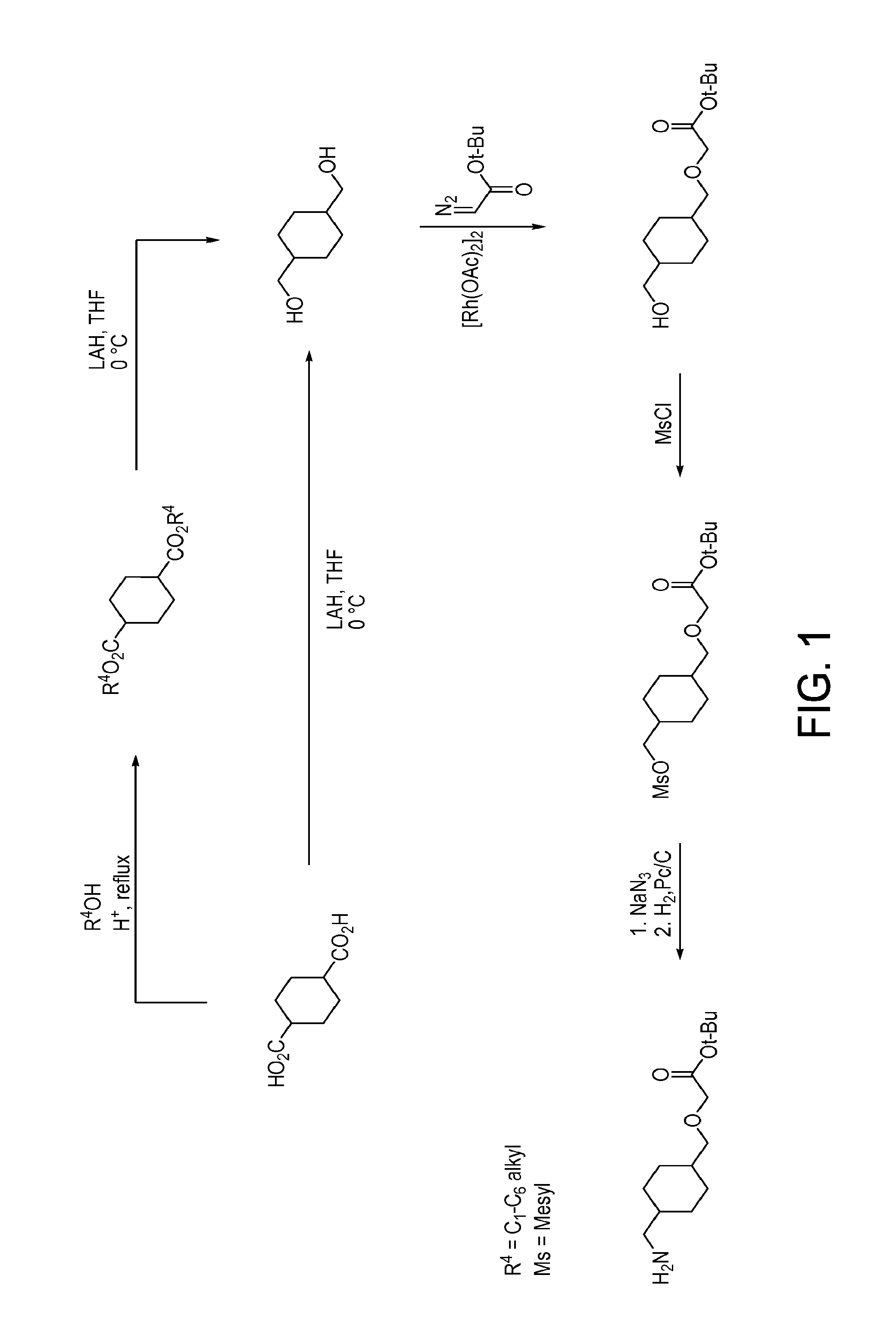
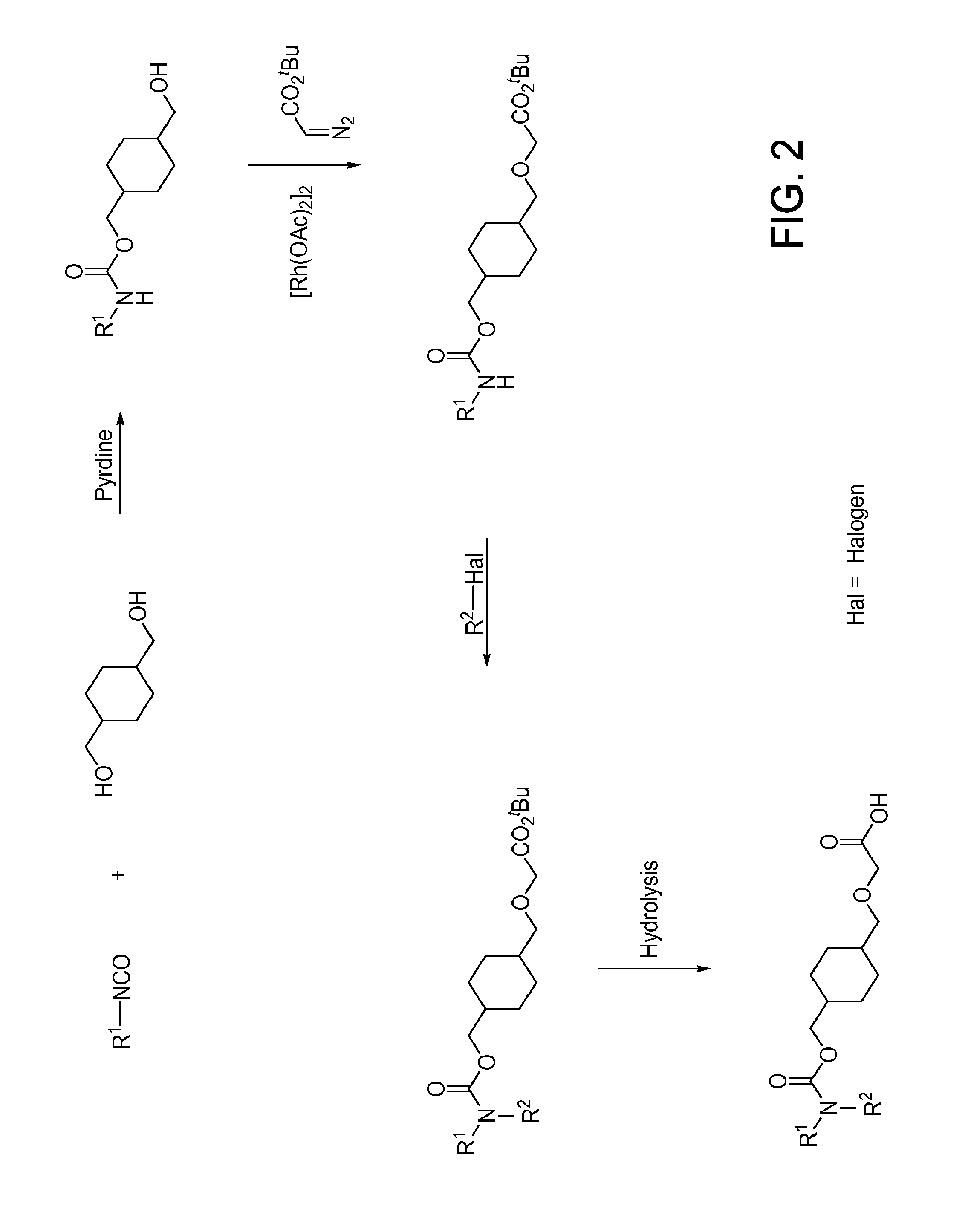
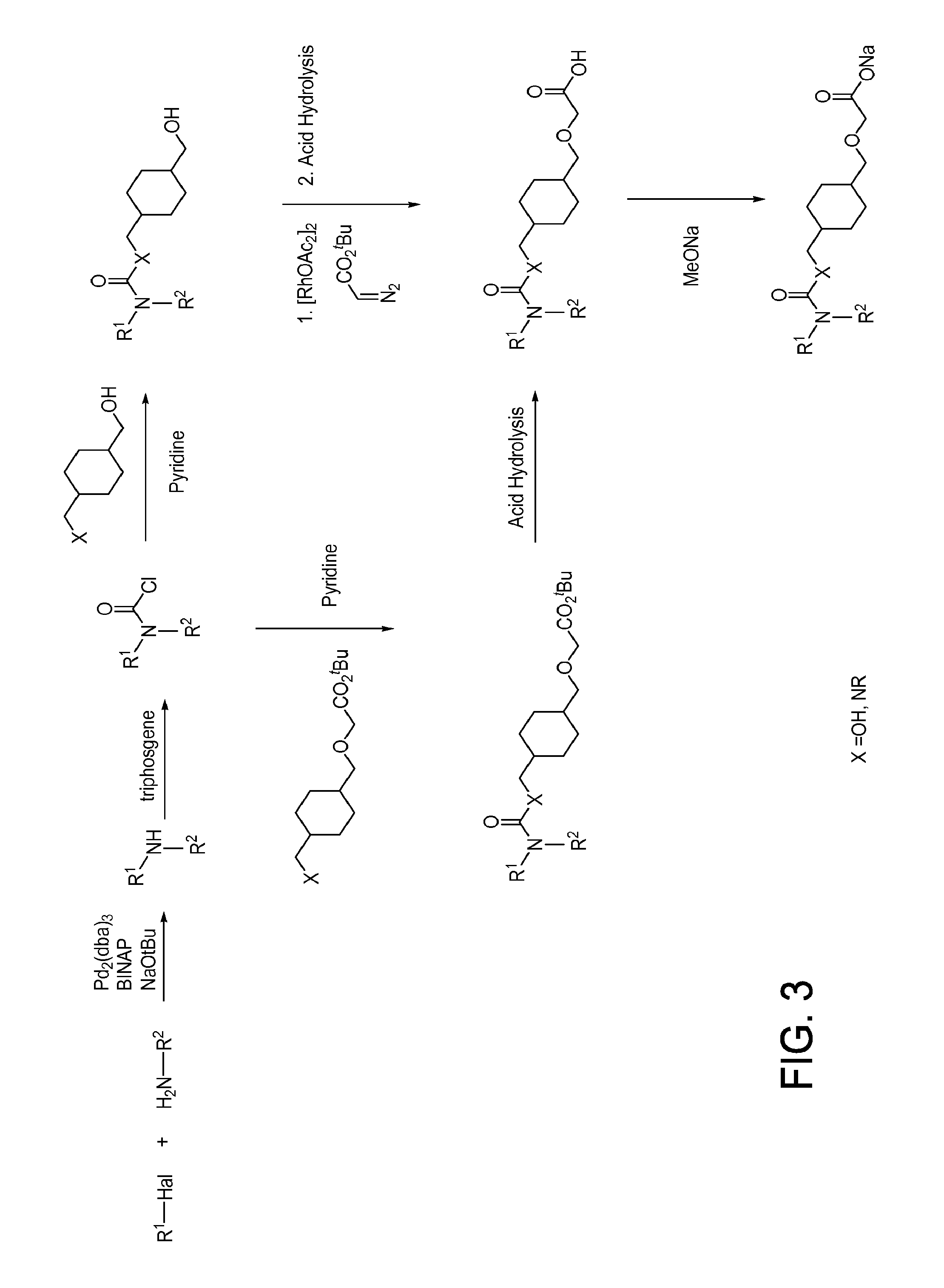
[1104]Illustrated syntheses for compounds of the present invention are shown in FIGS. 1 through 6 where the symbols have the same definitions as used throughout this disclosure.
[1105]The compounds of the invention and their syntheses are further illustrated by the following examples. The following examples are provided to further define the invention without, however, limiting the invention to the particulars of these examples. The compounds described herein, supra and infra, are named according to the CS ChemDraw Ultra Version 7.0.1, AutoNom version 2.2, or CS ChemDraw Ultra Version 9.0.7. In certain instances common names are used and it is understood that these common names would be recognized by those skilled in the art.
[1106]Chemistry:
[1107]Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a Bruker Avance-400 equipped with a QNP (Quad Nucleus Probe) or a BBI (Broad Band Inverse) and z-gradient. Chemical shifts are...
example 1.1
Preparation of tert-Butyl 2-(((1s,4s)-4-((Phenylcarbamoyloxy)methyl)cyclo-hexyl) methoxy)acetate
Step A: Preparation of (1s,4s)-Diethyl Cyclohexane-1,4-dicarboxylate
[1109]To a solution of (1s,4s)-cyclohexane-1,4-dicarboxylic acid (25 g, 145 mmol) in ethanol (150 mL) was added concentrated H2SO4 (98%, 1 mL). The reaction was heated to reflux for 16 h, cooled to room temperature and concentrated. The residue was extracted with EtOAc and saturated NaHCO3, washed with brine, dried over MgSO4, and filtered. The filtrate was concentrated to provide the title compound as colorless oil (30.5 g). 1H NMR (400 MHz, CDCl3) δ ppm 1.25 (t, J=7.14 Hz, 6H), 1.62-1.75 (m, 4H), 1.84-1.97 (m, 4H), 2.40-2.50 (m, 2H), 4.13 (q, J=7.12 Hz, 4H).
Step B: Preparation of (1s,4s)-Cyclohexane-1,4-diyldimethanol
[1110]To a solution of (1s,4s)-diethyl cyclohexane-1,4-dicarboxylate (13.0 g, 56.9 mmol) in THF (500 mL) was added lithium aluminum hydride (4.54 g, 120 mmol) in portions at 0° C. The mixture was stirred at...
example 1.2
Preparation of 2-(((1s,4s)-4-(((4-Methoxyphenyl)(phenyl)carbamoyloxy)methyl)cyclohexyl)methoxy)acetic Acid (Compound 24)
[1113]To a solution of tert-butyl 2-(((1s,4s)-4-((phenylcarbamoyloxy)methypeyclohexyl)methoxy)acetate (0.1 g, 0.265 mmol) in dioxane (2 mL) were added 1-iodo-4-methoxybenzene (0.062 g, 0.265 mmol), (1R,2R)-cyclohexane-1,2-diamine (0.030 g, 0.265 mmol), CuI (0.02 g, 0.158 mmol), and K3PO4 (0.1 g, 0.471 mmol) at room temperature. The reaction mixture was sealed in a reaction vial and heated to 150° C. under microwave irradiation for 4 h. The mixture was filtered and the filtrate was concentrated. The residue was treated with HCl (4.0 N in dioxane, 5 mL) for 16 h. The resulting mixture was concentrated and purified by preparative HPLC. LCMS m / z=428.2 [M+H]+; 1H NMR (400 MHz, DMSO-d6) δ ppm 1.34-1.56 (m, 8H), 1.70-1.88 (m, 2H), 3.31 (s, 3H), 3.39 (d, J=7.07 Hz, 2H), 4.00 (d, J=7.20 Hz, 2H), 4.11 (s, 2H), 6.94-7.01 (m, 2H), 7.23-7.31 (m, 4H), 7.42-7.49 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com