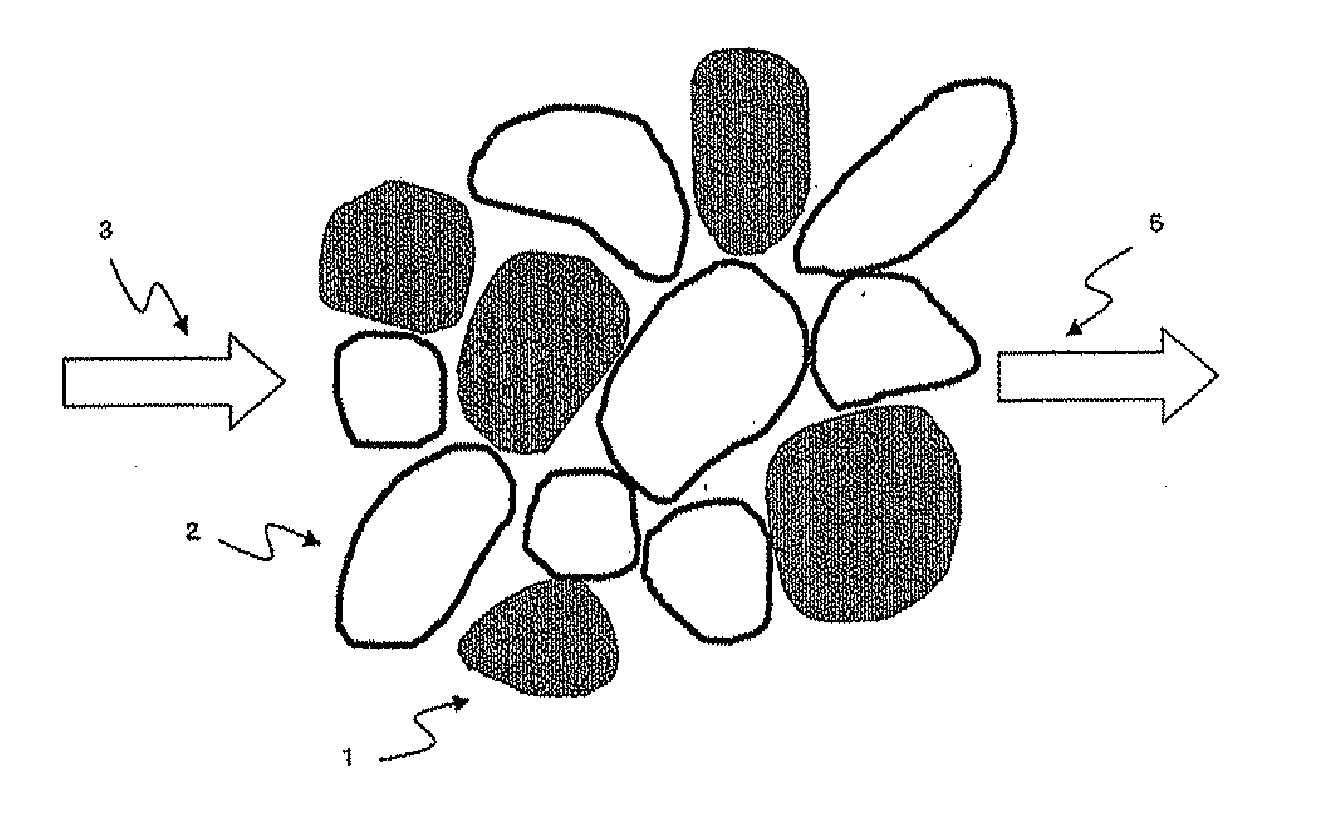
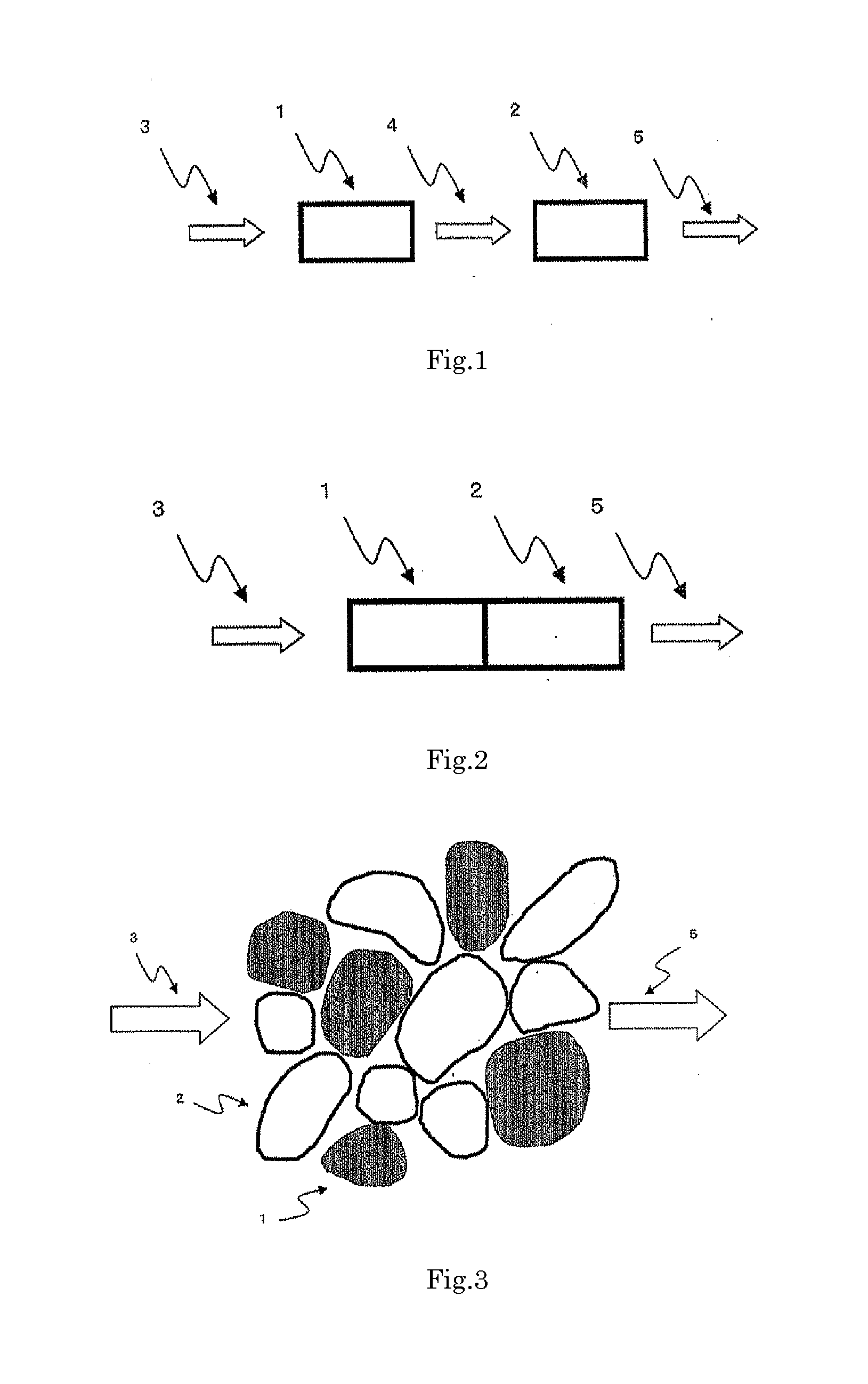
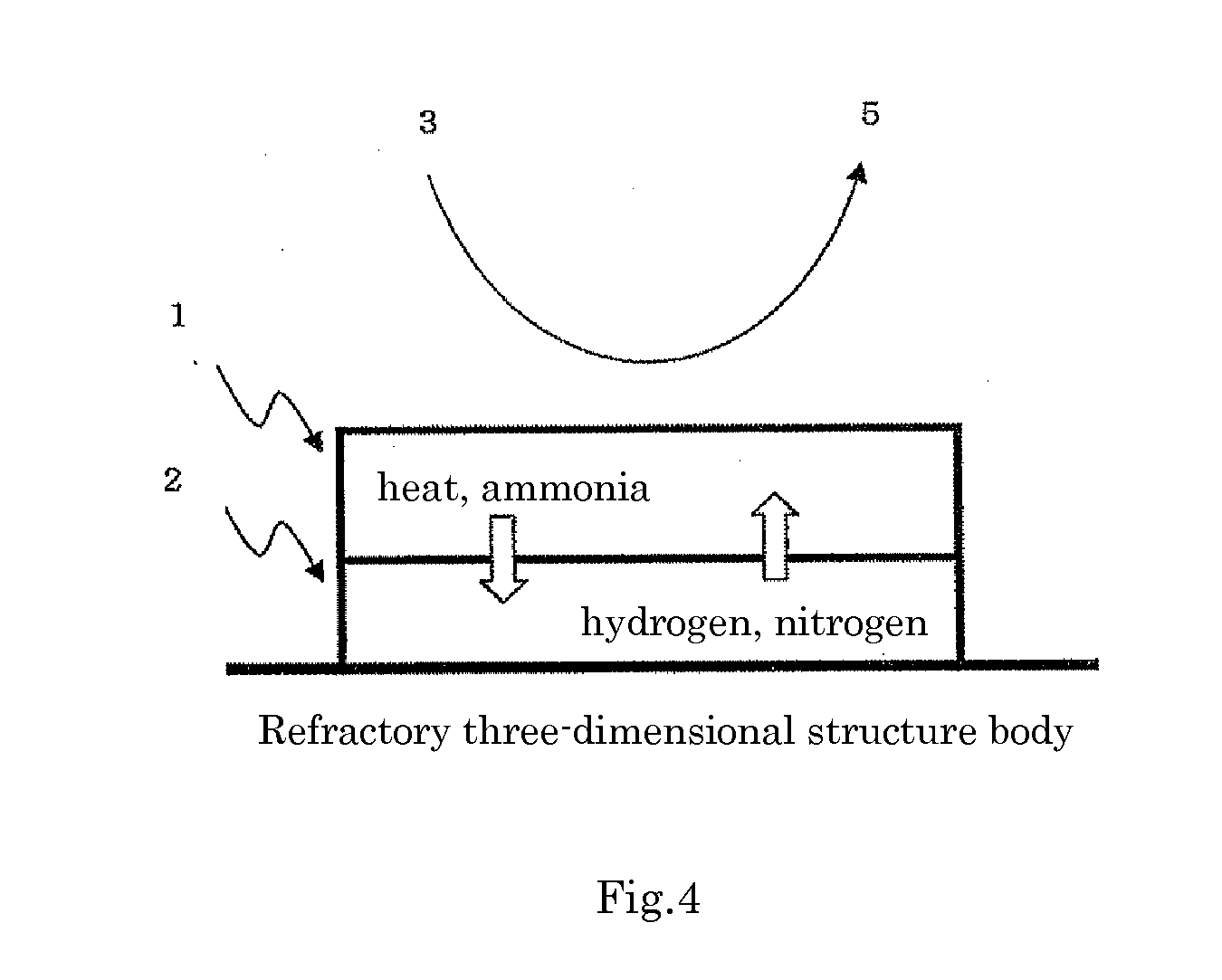
Catalyst for production of hydrogen and process for producing hydrogen using the catalyst, and catalyst for combustion of ammonia, process for producing the catalyst and process for combusting ammonia using the catalyst
a technology of catalysts and catalysts, which is applied in the direction of catalyst activation/preparation, metal/metal-hydroxide catalysts, catalysts/metal-hydroxide catalysts, etc., can solve the problems of difficult control of reaction, strong equipment dependence of hydrogen produced by such processes, and lack of convenience in the aspect of simple utilization, etc., to achieve efficient hydrogen production from ammonia, promote self-directing reaction, and cheap and practical catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example 1
[0113]An evenly and densely mixed manganese-cerium oxide, which is A component, was prepared by the following method, according to a solid-liquid mixing method. Powder-formed cerium carbonate and an aqueous solution of manganese nitrate were weighed and sufficiently mixed in such a manner that the content of manganese oxide in the evenly and densely mixed manganese-cerium oxide became 50% by mass in terms of MnO2, and the obtained mixture was dried at 150° C. overnight, fired at 500° C. for 5 hours and milled by a hammer mill to obtain the evenly and densely mixed manganese-cerium oxide powder. When the obtained evenly and densely mixed manganese-cerium oxide was subjected to the x-ray diffraction measurement in the condition of using a CuKα ray source, a voltage of 45 KV, a current of 40 mA, a scanning range of 10 to 90° and a scanning rate of 0.198° / minute, a main peak at the position showing the fluorite type crystal structure of cerium dioxide was detected and no crystal peak de...
experimental example 2
[0115]An aqueous solution of manganese-silver-lanthanum was prepared by adding 28.7 g of manganese nitrate hexahydrate, 4.25 g of silver nitrate and 54.1 g of lanthanum nitrate hexahydrate to 1000 mL of pure water. Next, while an aqueous TMAH solution, which was obtained by adding pure water to 1050 g of an aqueous TMAH solution of 25% by mass to dilute the solution to a liquid amount of about 3 L, being violently stirred, the aqueous solution of manganese-silver-lanthanum was slowly added dropwise thereto. On completion of the dropwise addition, the mixture was continuously stirred for about 30 minutes for aging. After the aging, the obtained precipitate was filtered, washed with pure water and thereafter dried at 110° C. The dried material was pulverized, and thereafter fired at 400° C. for 1 hour and further fired at 650° C. for 2 hours in atmospheric air to obtain a manganese-silver-lanthanum composite oxide (catalyst 2). When the catalyst 2 was subjected to the x-ray diffractio...
experimental example 3
[0116]A cerium carbonate powder in an amount of 19.6 g was added to an aqueous solution obtained by dissolving 6.6 g of manganese nitrate hexahydrate, 25.4 g of cobalt nitrate hexahydrate and 1.47 g of silver nitrate in distilled water. Next, the mixture was heated while being stirred by a hot stirrer for evaporating water therein to obtain a dried solid. The dried solid was dried at 150° C. overnight and thereafter, pulverized, and fired at 500° C. for 2 hours in atmospheric air to obtain a cobalt-silver-manganese-cerium oxide powder (catalyst 3). When the catalyst 3 was subjected to the x-ray diffraction measurement in the same conditions as those of Experimental Example 1, it was found that merely a peak showing the fluorite type crystal structure of cerium dioxide and a peak showing the structure of tricobalt tetroxide were detected, and no crystal peaks derived from silver and manganese were observed. Therefore, it was confirmed that manganese existed in the form of an evenly a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com