Poly(3-hydroxyalkanoate) resin composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
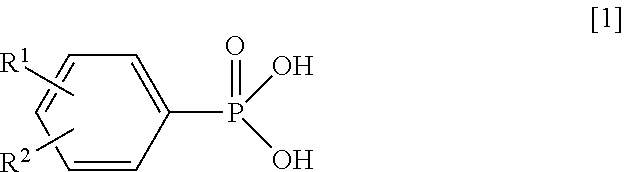
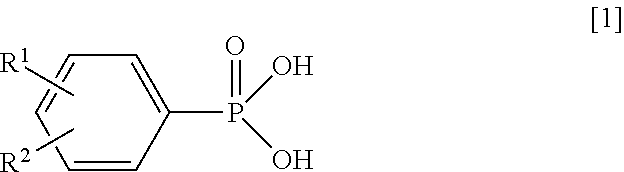
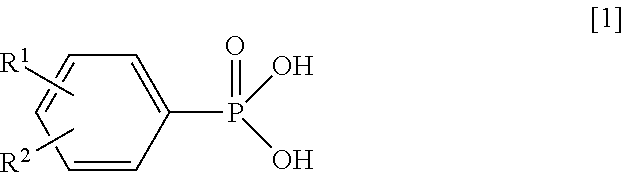
Synthesis of Magnesium Phenylphosphonate (PPA-Mg)
[0064]Into a reaction vessel equipped with a stirrer were charged 10.2 g (50 mmol) of magnesium chloride hexahydrate (manufactured by Wako Pure Chemical Industries, Ltd.) and 100 g of water, and the mixture was stirred to obtain a uniform solution. Next, to this solution being stirred at room temperature (about 25° C.), a solution in which 7.8 g (50 mmol) of PPA and 4.2 g (105 mmol) of sodium hydroxide had been dissolved in 68 g of water was added, and the resultant solution was stirred for additional 1 hour. The produced solid was filtered by vacuum filtration and was washed with water. The resultant wet product was dried at 200° C. for 6 hours to obtain target magnesium phenylphosphonate as white powder.
synthesis example 2
Synthesis of Calcium Phenylphosphonate (PPA-Ca) (1)
[0065]Operating in the same manner as in Synthesis Example 1 except that 7.4 g (50 mmol) of calcium chloride dihydrate (manufactured by Wako Pure Chemical Industries, Ltd.) was used in place of magnesium chloride hexahydrate, target calcium phenylphosphonate (1) was obtained as white powder.
[0066]A scanning electron microscope (SEM) [JSM-7400F manufactured by JEOL Ltd.] image of the resultant powder was observed. Particles of the powder were strip-shaped, and the average of the approximately maximum minor axis (where the size of the strip-shaped particle is represented by , and these figures satisfy ) of 50 particles extracted at random was about 1 μm.
synthesis example 3
Synthesis of Manganese Phenylphosphonate (PPA-Mn)
[0067]Operating in the same manner as in Synthesis Example 1 except that 9.9 g (50 mmol) of manganese chloride tetrahydrate (manufactured by Wako Pure Chemical Industries, Ltd.) was used in place of magnesium chloride hexahydrate, target manganese phenylphosphonate was obtained as pale pink powder.
[0068]The resultant manganese phenylphosphonate, which was an anhydride immediately after being dried, changed to a monohydrate in an air atmosphere at room temperature (about 25° C.).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Crystallization enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com