Method of weight management
a weight management and weight technology, applied in the field of life-threatening disorders, can solve the problems of increasing the risk of morbidity and mortality, increasing the risk of death from a variety of causes, and increasing the risk of obesity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Phase 3 Studies
[0399]APD356-009 (“BLOOM”) was a 104-week, placebo controlled study that assessed the safety and efficacy of lorcaserin 10 mg BID in overweight and obese patients, with concurrent behavior modification. The primary efficacy objective during Year 1 was to evaluate weight loss; the primary objective during Year 2 was to assess the ability of lorcaserin to maintain body weight loss that was achieved during Year 1. At study start, each patient received randomized, double blind treatment assignments for Year 1 and for Year 2 (all patients were given a new randomization number for Year 2 to assure that patients and study personnel remained blinded to treatment assignments). All patients assigned to placebo during Year 1 (50% of randomized population) remained on placebo in Year 2. Patients assigned to lorcaserin during Year 1 were randomly assigned to stay on lorcaserin during Year 2 (⅔ of Year 1 lorcaserin patients) or to switch to placebo (⅓ of Year 1 lorcaserin patients)...
example 2
Identifying Responders Based on Early Weight Loss
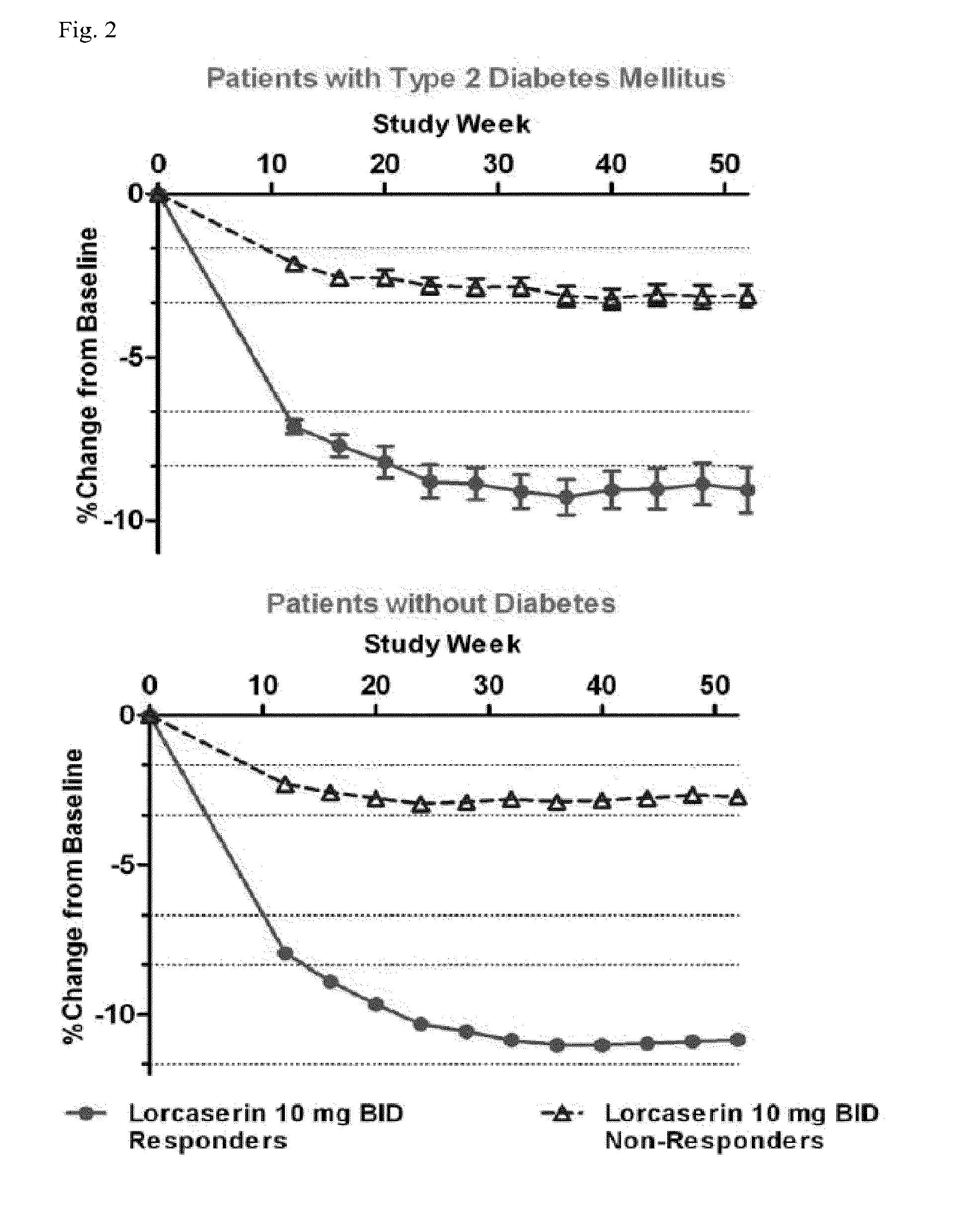
[0402]An analysis of early weight loss was performed, at each visit after baseline through Week 24, as a predictor of ultimate weight loss success, as defined by achieving at least about 5% weight loss at 52 weeks, or at least about 10% weight loss at 52 weeks. The area under the curve (AUC) for the receiver operating characteristic (ROC) curve measures the balance between specificity and sensitivity by measuring how often a predictor (e.g., Week 12 or Week 24 percent weight loss) and a successful outcome (e.g., at least 5% or 10% weight loss at Week 52) are concordant.
[0403]At Week 12, the optimal thresholds were 4.6% and 5.9% weight loss for predicting at least 5% and at least 10% weight loss at Week 52. At Week 24, the optimal weight loss thresholds were 6.1% and 8.5%, respectively (Table 1 and Table 2). Non-diabetic patients who achieved at least 5% weight loss at Week 12, lost on average approximately 10.6 kilos (23 pounds) at We...
example 3
Week 52 Outcomes for Those Achieving at Least 5% Weight Loss at Week 12
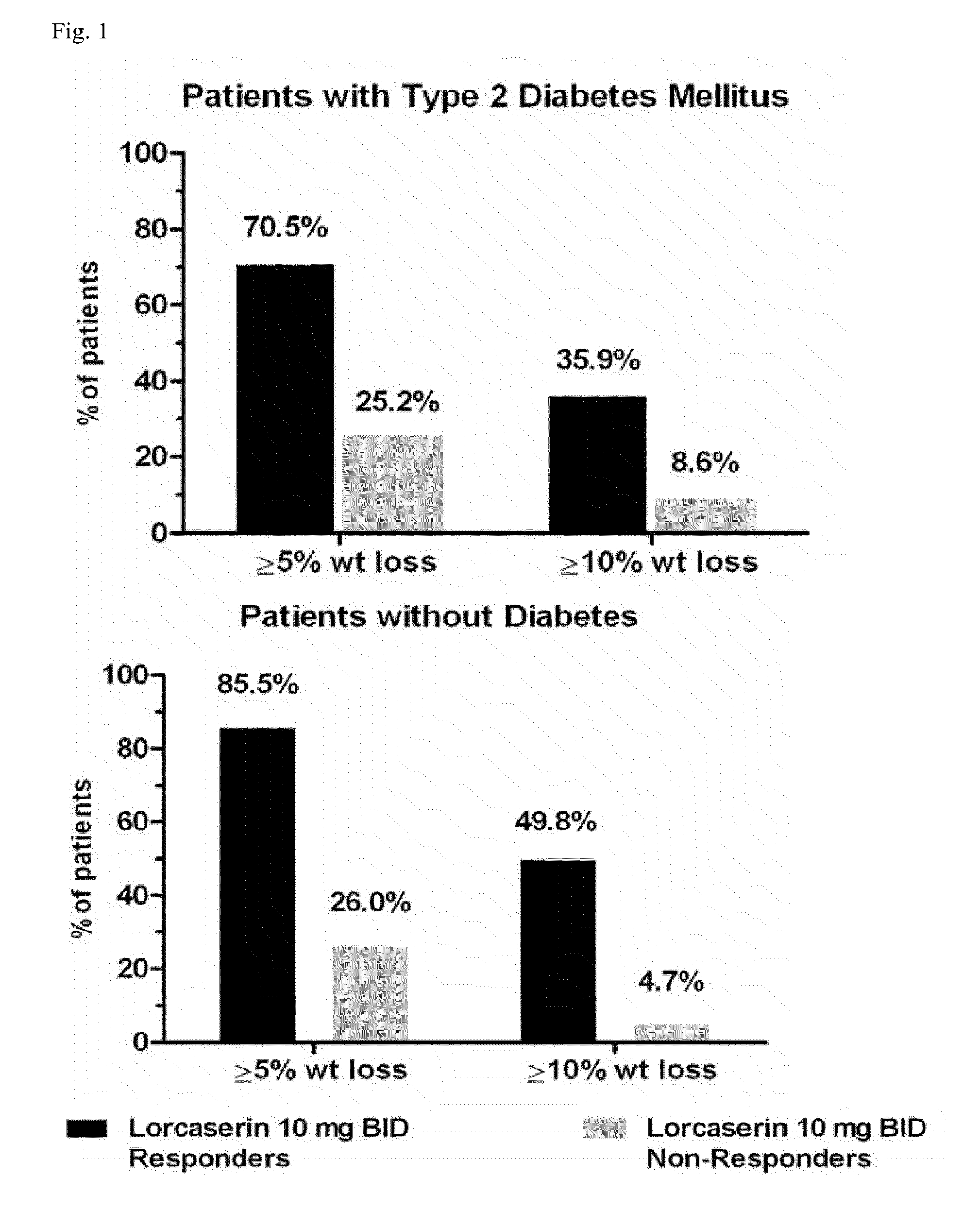
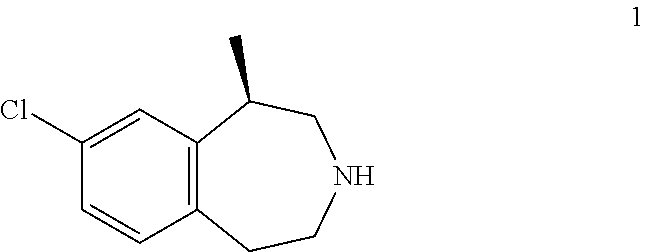
[0406]In patients without type 2 diabetes mellitus (T2DM), proportions achieving ≧5% weight loss and weight loss at Week 52 for (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride salt hemihydrate (“drug”) and placebo patients were 47 vs. 23% and 5.8 vs. 2.5 kg, respectively (MITT-LOCF). Results in patients with T2DM were 38 vs. 16% and 4.7 vs. 1.9 kg, respectively. Proportions meeting the ≧5% weight loss at Week 12 criterion were 49.3% and 35.9% for drug patients without and with T2DM, respectively, and 22.6% and 11.5% for placebo patients. Week 52 weight loss in non-diabetic drug Week 12 responders was 10.6 kg (23 lbs), and 86% and 50% of patients achieved at least 5% and 10% weight loss. In diabetic drug Week 12 responders, results were 9.3 kg (20 lbs), 71%, and 36%.
[0407]Week 52 reductions in FPG and A1C in diabetic drug Week 12 responders were 29.3 mg / dL and 1.2%, and were 7.8 mg / dL and 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com