Devices, systems and methods for monitoring hip replacements
a technology for hip replacement and monitoring devices, applied in the field of hip replacements, can solve the problems of improper operation of hip joint, patient discomfort, and various complications, and achieve the effect of being easily incorporated into bone cement or
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
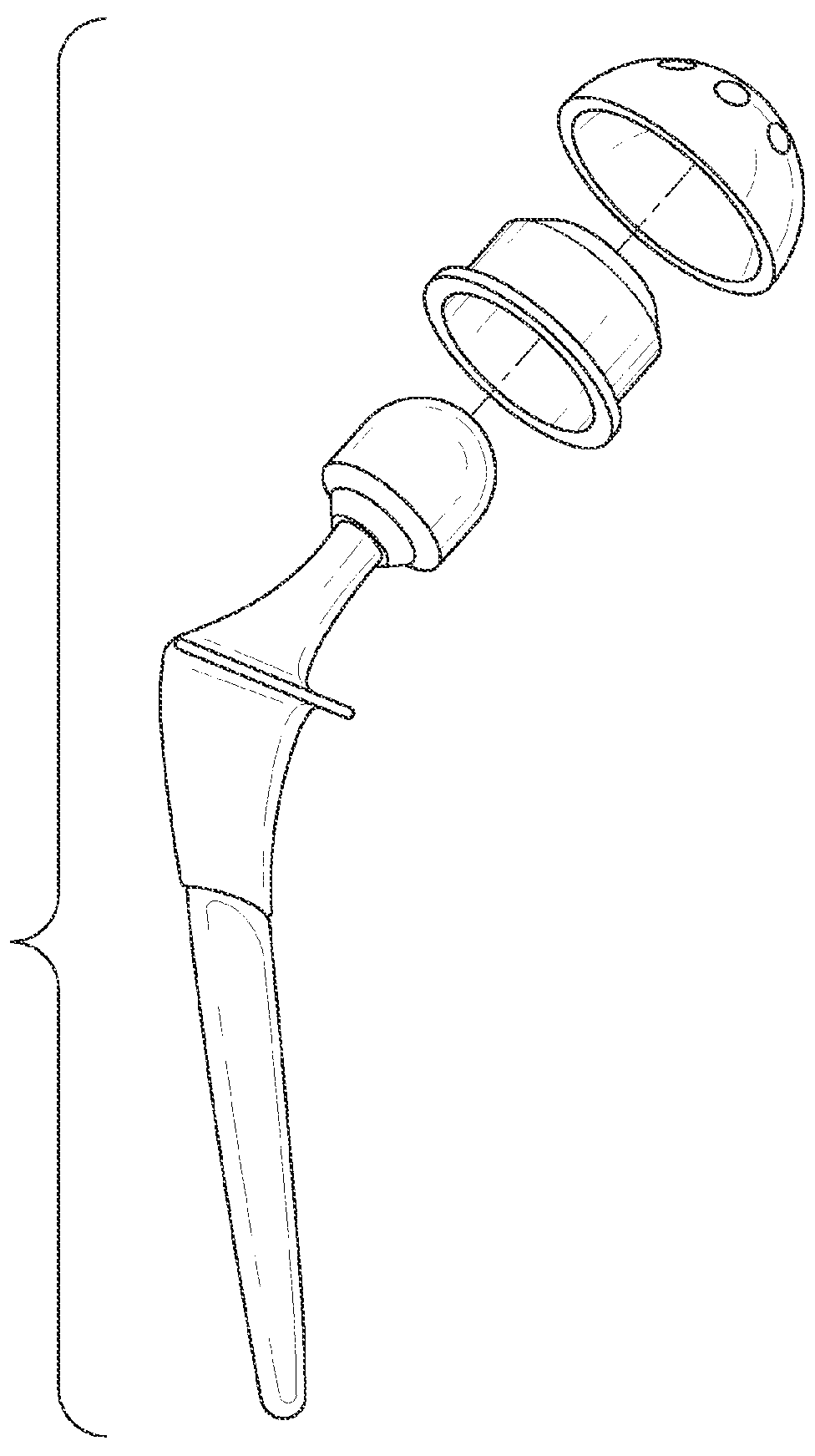
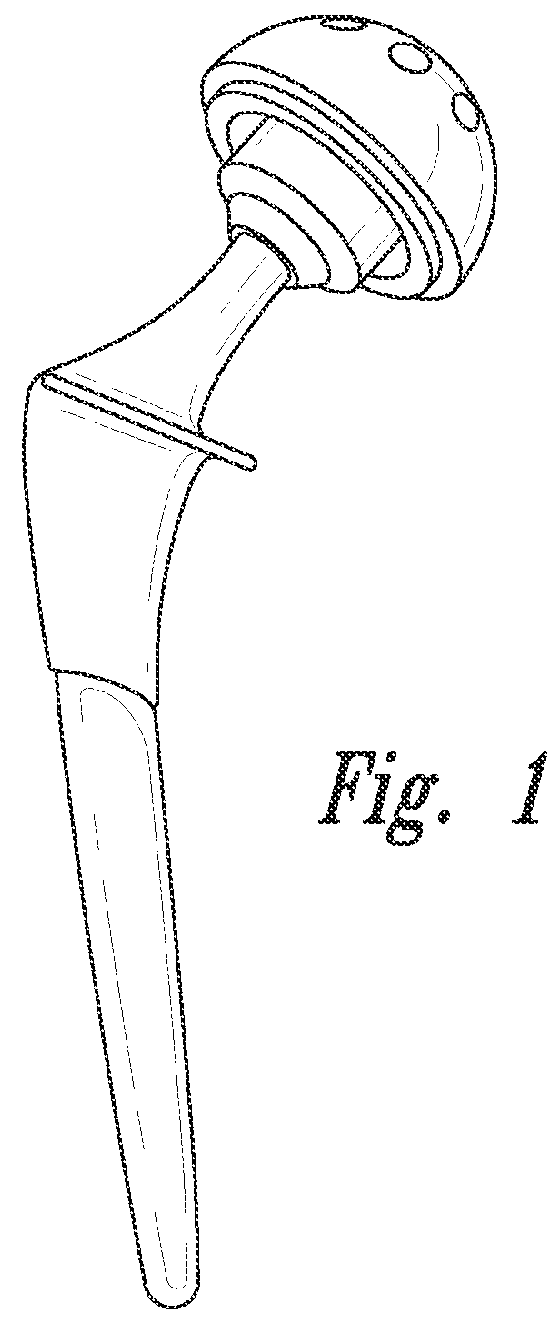
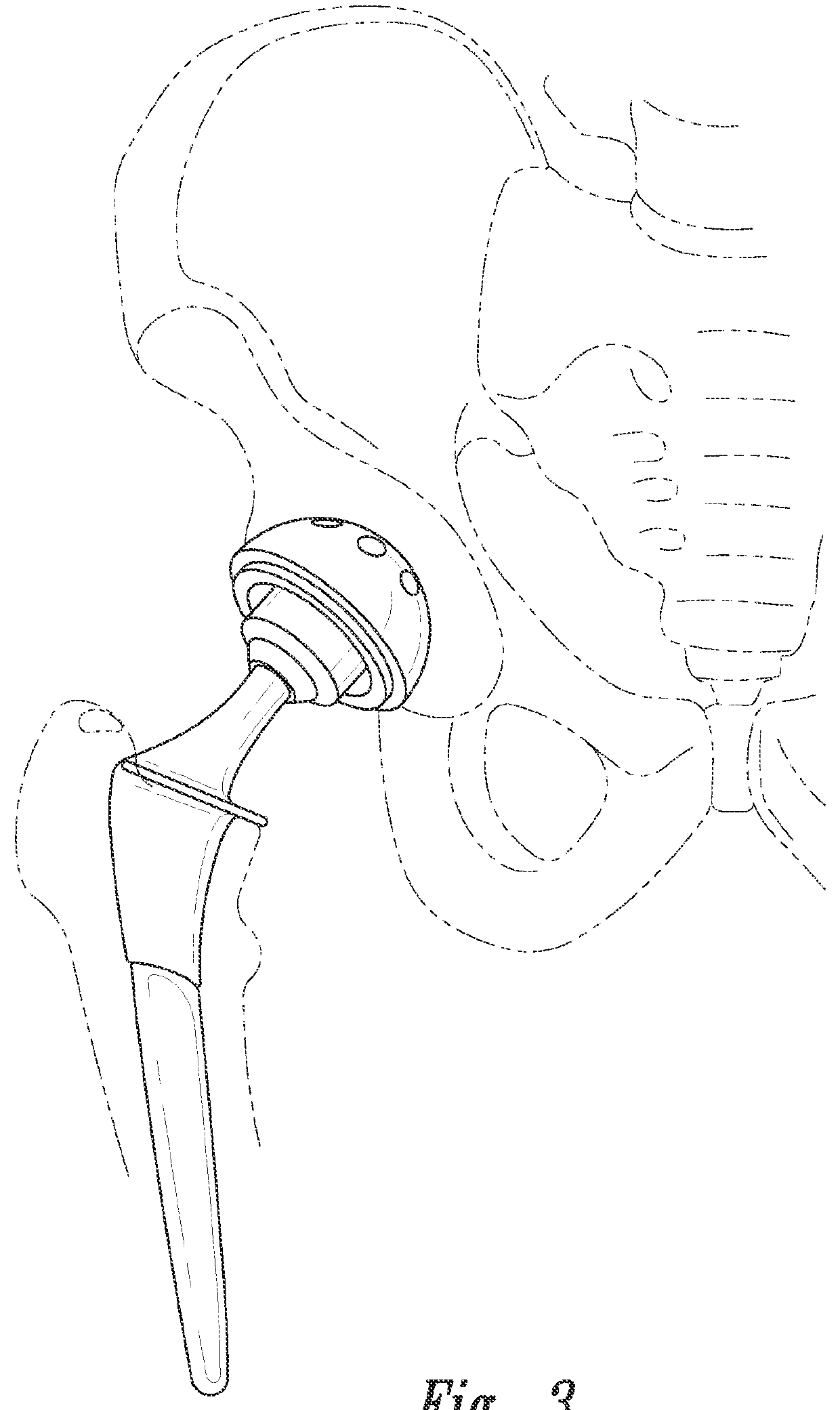
[0132]2) The hip replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a sensor on the femoral stem.
[0133]3) The hip replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a sensor on the femoral head.
[0134]4) The hip replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a sensor on the acetabular assembly.
[0135]5) The hip replacement prosthesis according to any one of embodiments 1 to 4 wherein said sensor is selected from the group consisting of accelerometers, pressure sensors, contact sensors, position sensors, chemical microsensors, tissue metabolic sensors, mechanical stress sensors and temperature sensors.
embodiment 5
[0136]6) The hip replacement prosthesis wherein said accelerometer detects acceleration, tilt, vibration, shock and or rotation.
[0137]7) The hip replacement prosthesis of embodiment 1 wherein the plurality of sensors includes contact sensors positioned between the femoral head and the acetabular assembly.
[0138]8) The hip replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a plurality of contact sensors positioned on the outer surface of the acetabular assembly.
[0139]9) The hip replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a plurality of contact sensors positioned on the outer surface of the acetabular assembly.
[0140]10) The hip replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a plurality of strain sensors positioned between the femoral head the acetabular assembly.
[0141]11) The hip replacement prosthesis of embodiment 1 wherein the plurality of sensors includes accelerometers positioned ...
embodiment 7
[0143]13) The hip replacement prosthesis of embodiment 7 further including strain sensors positioned between the acetabular liner and the acetabular shell.
[0144]14) A medical device, comprising a femoral stem and a plurality of sensors coupled to said femoral stem.
[0145]15) A medical device, comprising a femoral head and a plurality of sensors coupled to said femoral head.
[0146]16) A medical device, comprising an acetabular assembly and a plurality of sensors coupled to said acetabular assembly.
[0147]17) The medical device according to any one of embodiments 14 to 16, wherein said sensors are placed within and on the surface of said medical device.
[0148]18) The medical device according to any one of embodiments 14 to 17 wherein said sensor is selected from the group consisting of accelerometers, pressure sensors, contact sensors, position sensors, chemical microsensors, tissue metabolic sensors, mechanical stress sensors and temperature sensors.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com