Formulations with reduced oxidation
a technology of oxidation and formulation, applied in the field of protein formulations, can solve the problems of affecting the biological activity of the protein, unexpected and undesired consequences, and oxidation of amino acid residues, so as to prevent oxidation of the protein, reduce the oxidation potential, and reduce the effect of photosensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Antioxidant L-Trp Produces ROS that Oxidize Monoclonal Antibodies in Protein Formulations
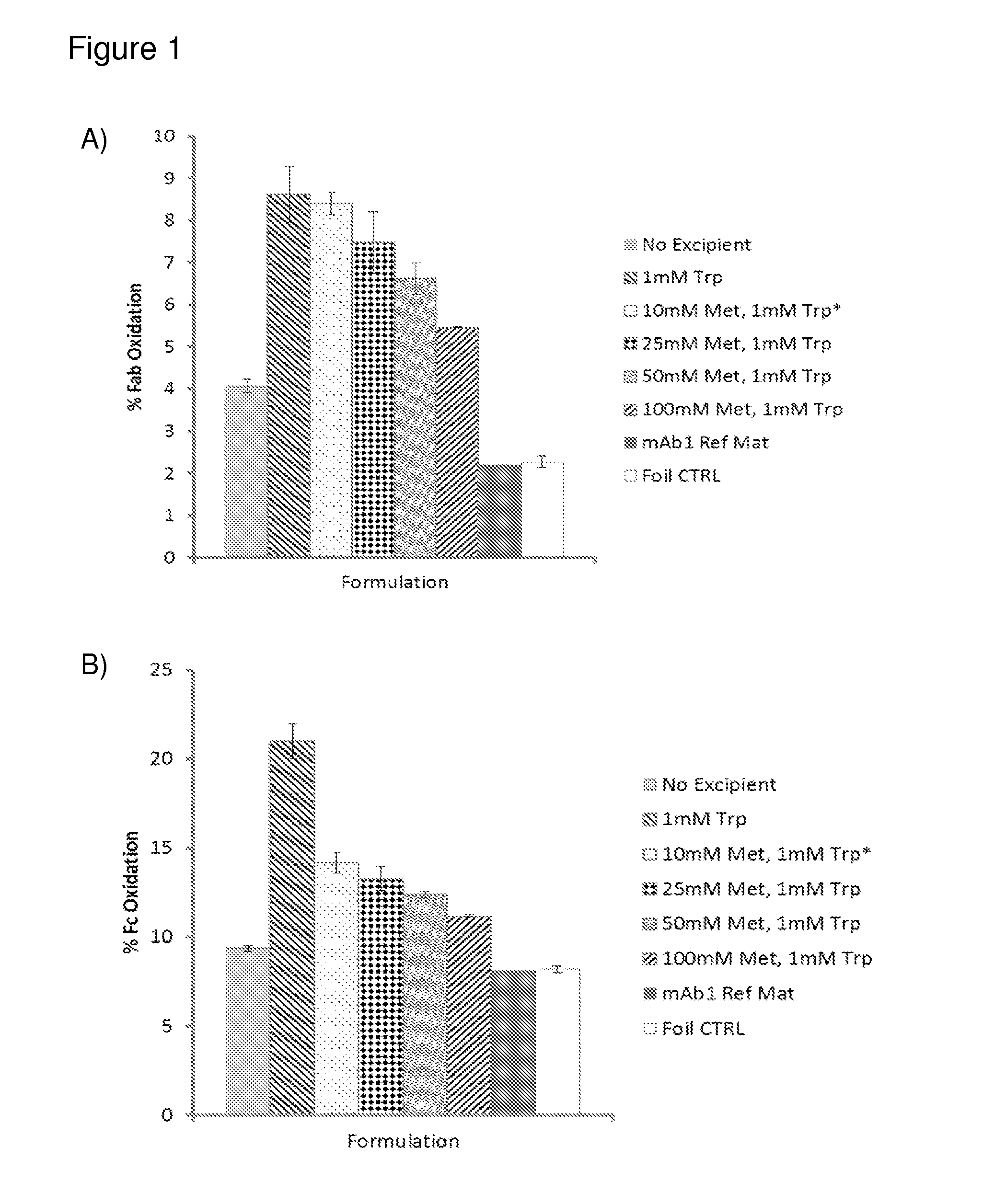
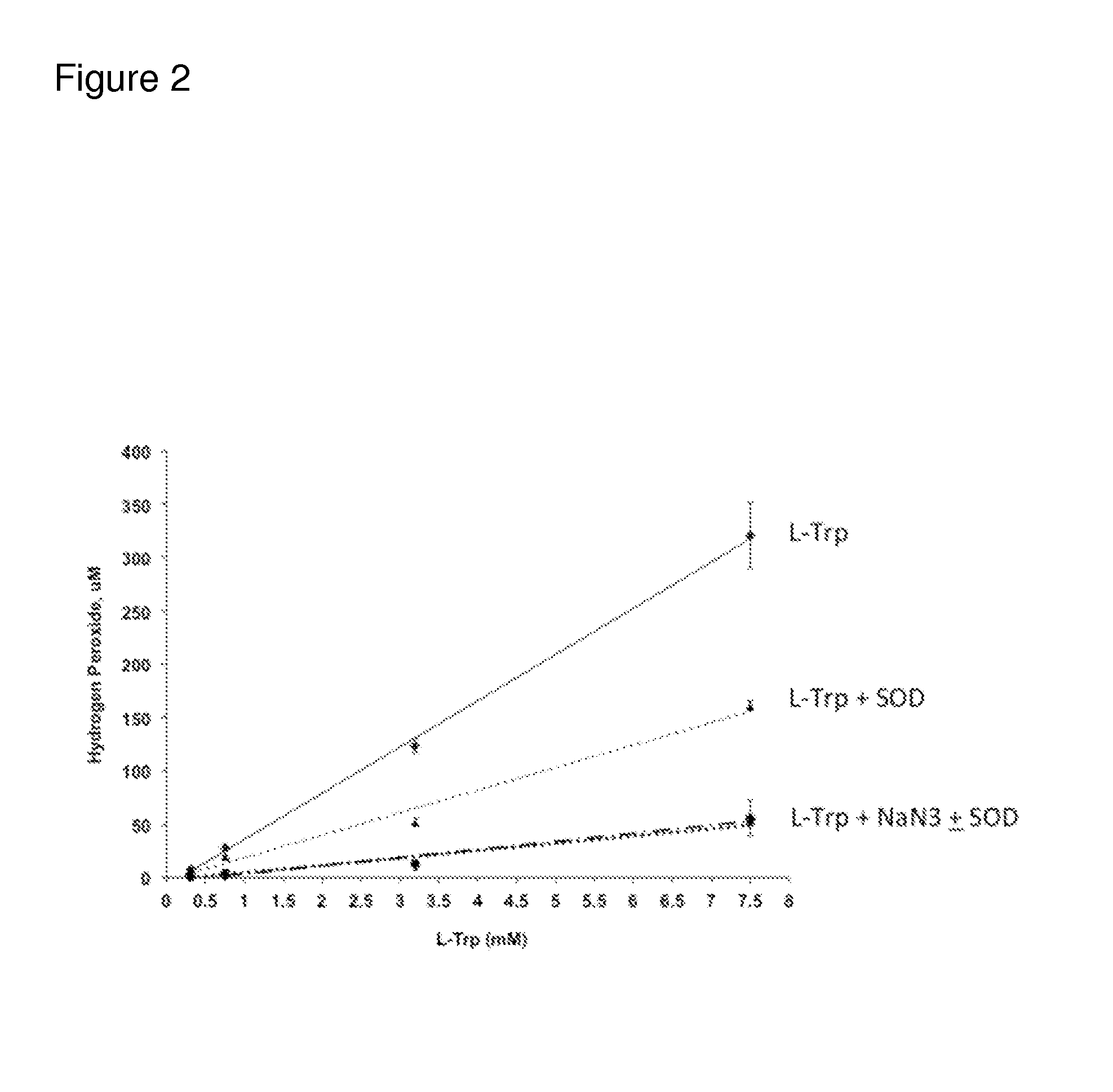
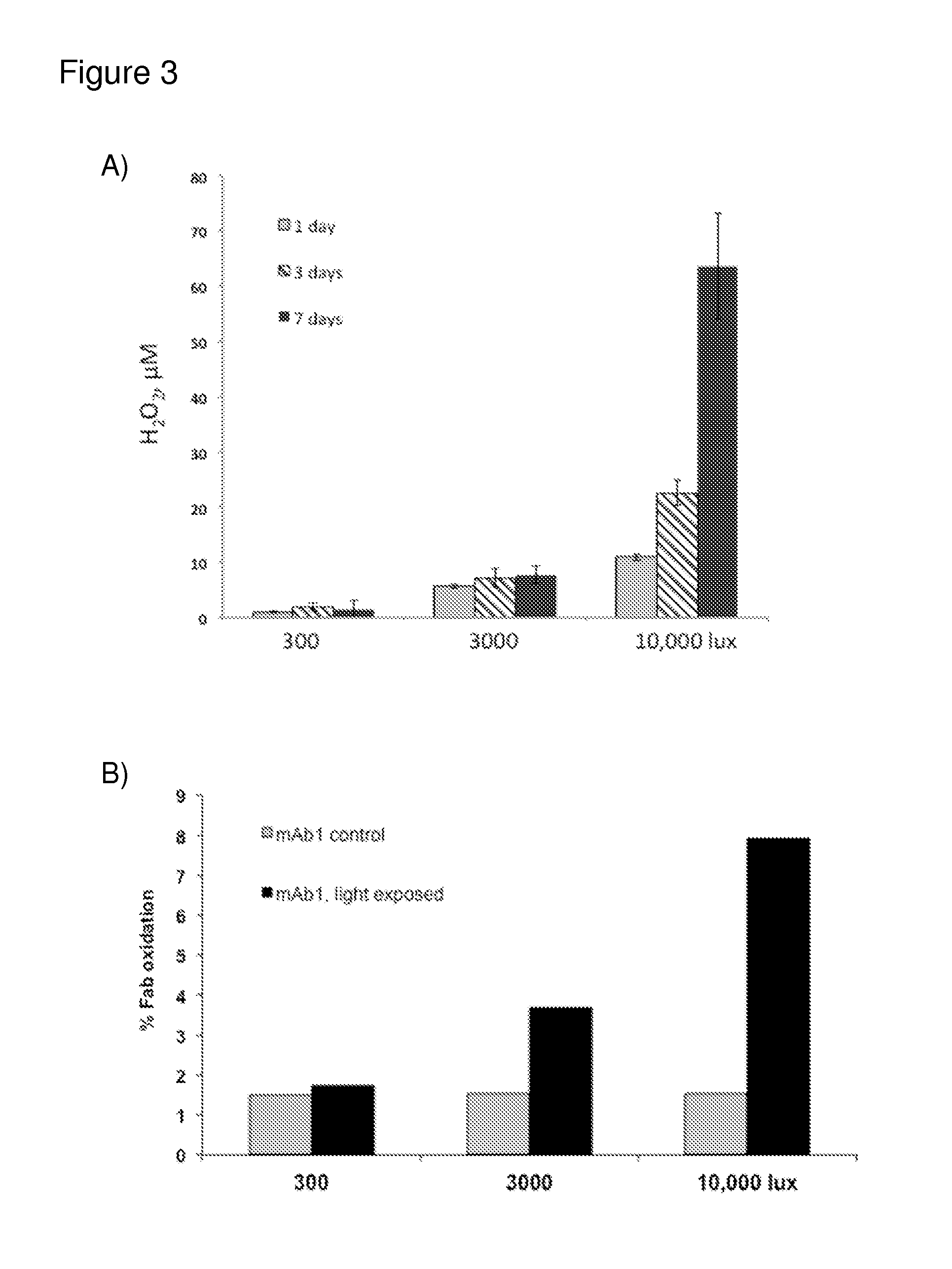
[0264]Monoclonal antibodies have been shown to produce ROS through the antibody catalyzed water oxidation pathway (ACWOP) wherein antibodies potentially catalyze a reaction between water and singlet oxygen generating hydrogen peroxide (Wentworth et al., Science 293(5536):1806-11 (2001); Wentworth et al., Proc Natl Acad Sci USA 97(20):10930-5 (2000)). In the ACWOP, a variety of ROS, including superoxide anion, dihydrogen trioxide, ozone, and even hydrotrioxy radical are generated in the pathway toward production of hydrogen peroxide (Zhu et al., Proc Natl Acad Sci USA 101(8):2247-52 (2004)). It has been shown that surface exposed tryptophans in a monoclonal anti-DR5 antibody, drozitumab (CAS number 912628-39-8), also referred to herein as mAb1, act as substrate (1O2 and O2−) generators that facilitate ACWOP even under mild light conditions in a time and concentration dependent manner (Sreedha...
example 2
Screening of Candidate Antioxidant Compounds
[0270]Tryptophan (Trp) is an electron rich amino acid that undergoes oxidative and electrophilic addition reactions in the presence of ROS such as hydroxyl radicals and singlet oxygen. Any potential antioxidant to protect Trp oxidation in proteins should have similar if not superior reactivity towards these ROS. A series of compounds that were either based on the L-Trp structure or have been reported to have antioxidant properties were evaluated. Compounds screened for antioxidant ability in this study included derivatives of tryptophan, indole, aromatic acids such as salicylic acid and anthranilic acid, and some vitamins. The chemical structures of the various compounds used were based on (A) Tryptophan derivatives (B) Kynurenine (C) Indole derivatives and (D) Aromatic acid derivatives:
(A) Tryptophan Derivatives
[0271]
NameRXAL-TryptophanCOOHHH5-Hydroxy-TryptophanCOOHOHH5-Methoxy-TryptophanCOOHOCH3H5-Amino-TryptophanCOOHNH2H5-Fluoro-Tryptop...
example 3
Candidate Antioxidant Compounds Reduced Oxidation of Monoclonal Antibody Formulations
[0284]Compounds that, compared to L-Trp, produced less H2O2 upon light treatment as well as those with lower oxidation potentials than L-Trp were chosen for evaluation of for their possible antioxidant properties using AAPH, light, and Fenton reaction as oxidative stress models (Table 3). mAb1 was used as a model protein to evaluate the effectiveness of select candidate antioxidants to protect against Trp oxidation by the different oxidation stress models. Each stress model produced oxidation through a different mechanism and therefore each was valuable in the assessment of biopharmaceutical stability. AAPH, or 2,2′-Azobis(2-Amidinopropane)Dihydrochloride, is used as a stress model to mimic alkyl peroxides potentially generated from formulation excipients such as degraded polysorbate. Decomposition of AAPH generates alkyl, alkoxyl, and alkyl peroxyl radicals that have been shown to oxidize amino aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com