SYSTEMIC DELIVERY OF MYOSTATIN SHORT INTERFERING NUCLEIC ACIDS (siNA) CONJUGATED TO A LIPOPHILIC MOIETY
a technology of interfering nucleic acids and lipophilic moiety, which is applied in the direction of genetic material ingredients, muscular disorders, drug compositions, etc., can solve the problems of reducing the usefulness of therapeutic applications, limiting the systemic sirna delivery to particulars, and affecting so as to reduce the expression of myostatin and reduce the level of myostatin protein. , the effect of reducing the expression of a myostatin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Mtsn siRNA
[0177]siRNA Synthesis—
[0178]siRNAs were synthesized by methods similar to those previously described (Wincott, F. et al., 1995, Nucleic Acids Res. 23:2677-84). For each oligonucleotide duplex, the individual, complementary sense and antisense strands were first synthesized on solid support, such as on controlled pore glass, using commercially available automated oligosynthesizers. The solid support was obtained pre-loaded with the first (3′) nucleotide unit of the desired sequence and placed in an appropriate column for the oligosynthesizer. The first nucleotide was linked to the solid support via a succinate linkage and contained a suitable acid sensitive protecting group (trityl, dimethoxytrityl) on the 5′-terminal hydroxyl group. The solid-phase oligosynthesis employed synthetic procedures that are generally known in the art. Elongation of the desired oligomeric sequence went through a cycle of four steps: 1) Acidic deprotection of the 5′-trityl protecting group; 2) Cou...
example 2
Mtsn siRNA Cholesterol Conjugates
[0192]Cholesterol Conjugation—
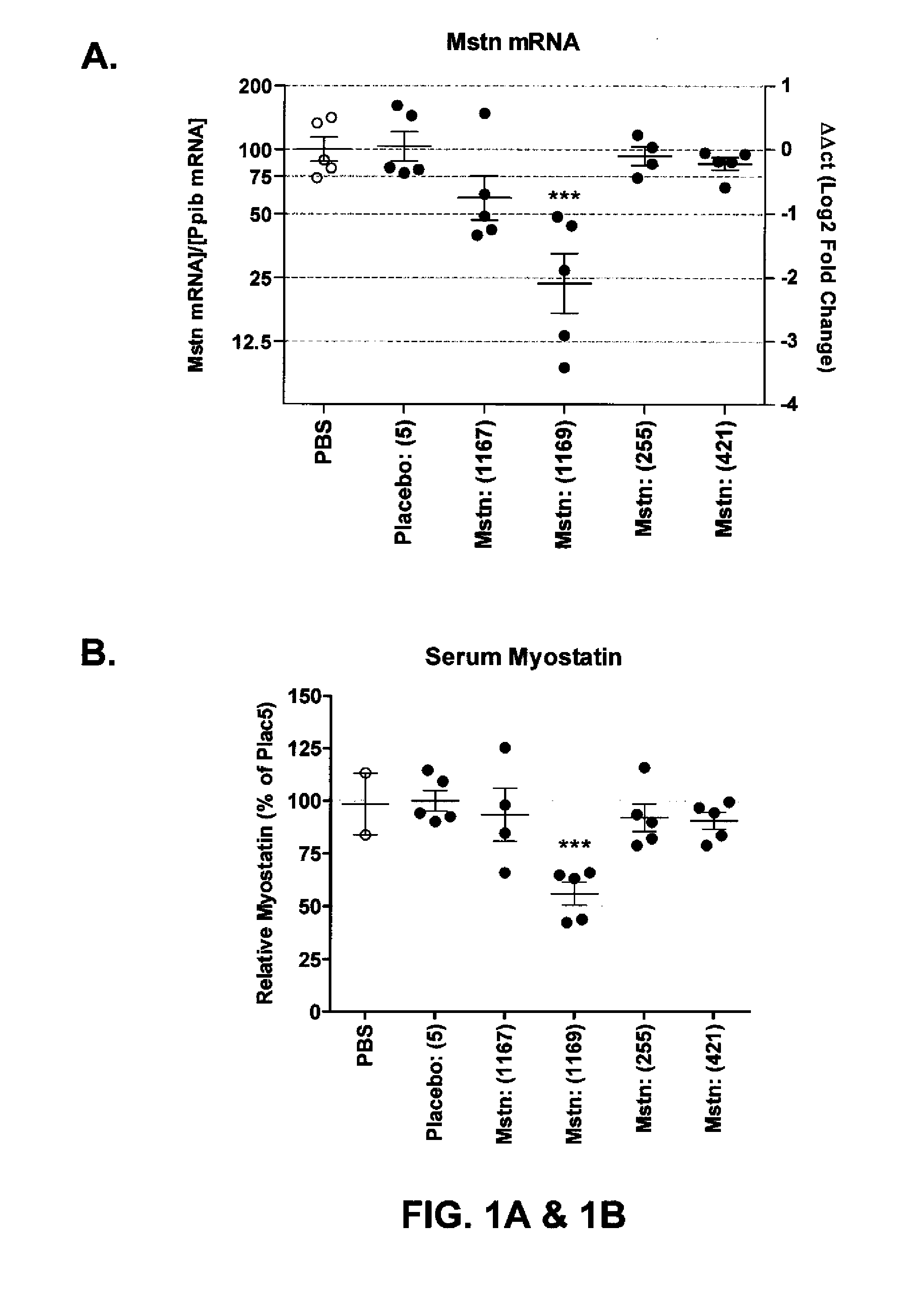
[0193]A single cholesterol entity was attached to the 3′ end of the sense strand of each siRNA molecule described in Table 3. For the preparation of oligonucleotides with a cholesterol unit on the 3′-terminus, deoxycytidylyl-deoxyguanosine (CpG) (see Table 6d, infra, for structure) with a preloaded cholesterol succinate, which also contained a dimethoxytrityl (DMT) protected primary alcohol, was used for synthesis of the corresponding oligonucleotide sequence (see Example 1). Typically, the final 5′-DMT protecting group was removed during oligosynthesis. The oligonucleotide was then cleaved from the CpG by treatment with a basic solution, such as aqueous methylamine, and purified by chromatography with a reversed phase resin, such as C18. This purified oligo was annealed to its corresponding complementary strand to prepare the desired oligonucleotide duplex.
[0194]Animals—
[0195]Female CD-1 mice were obtained from Charles ...
example 3
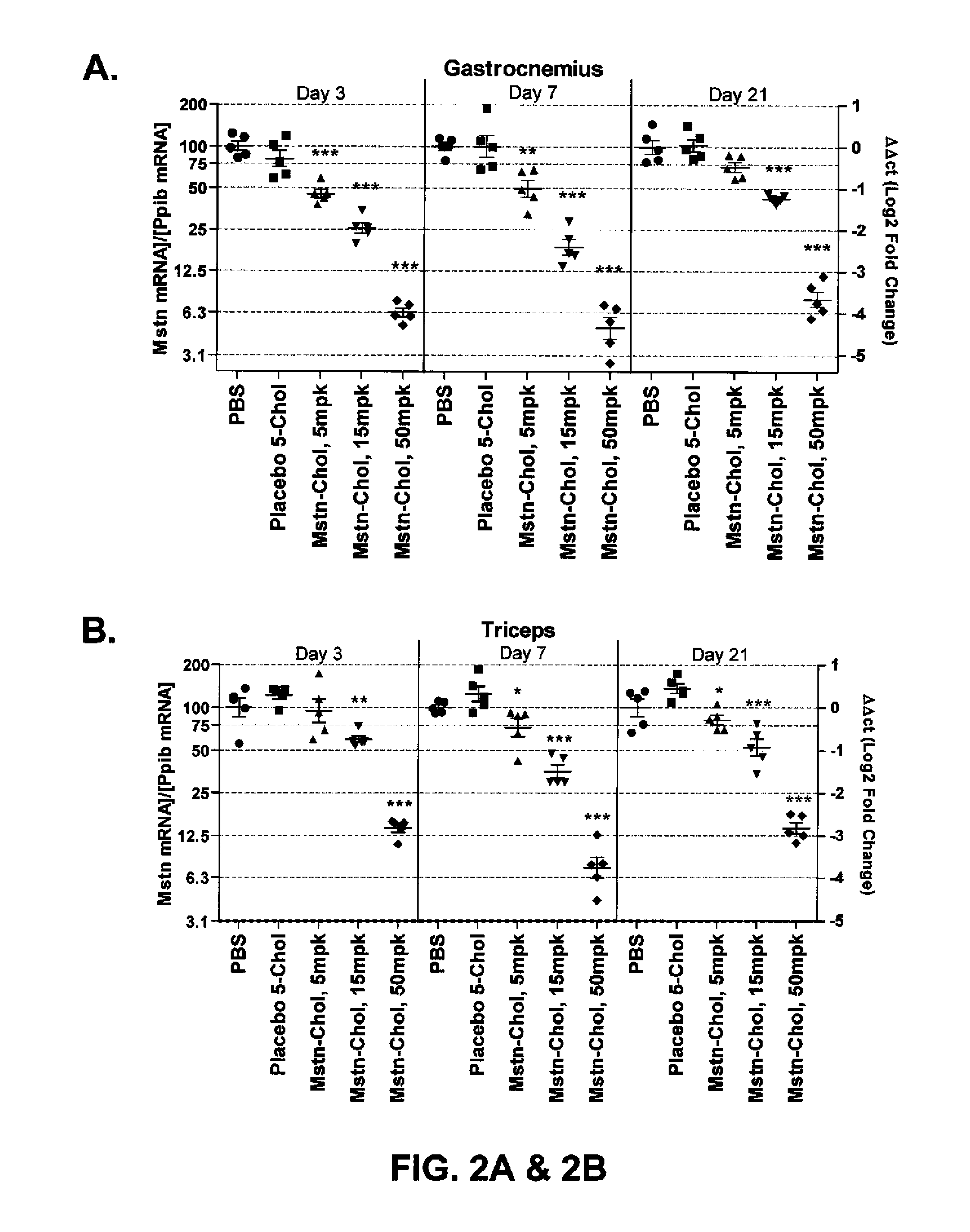
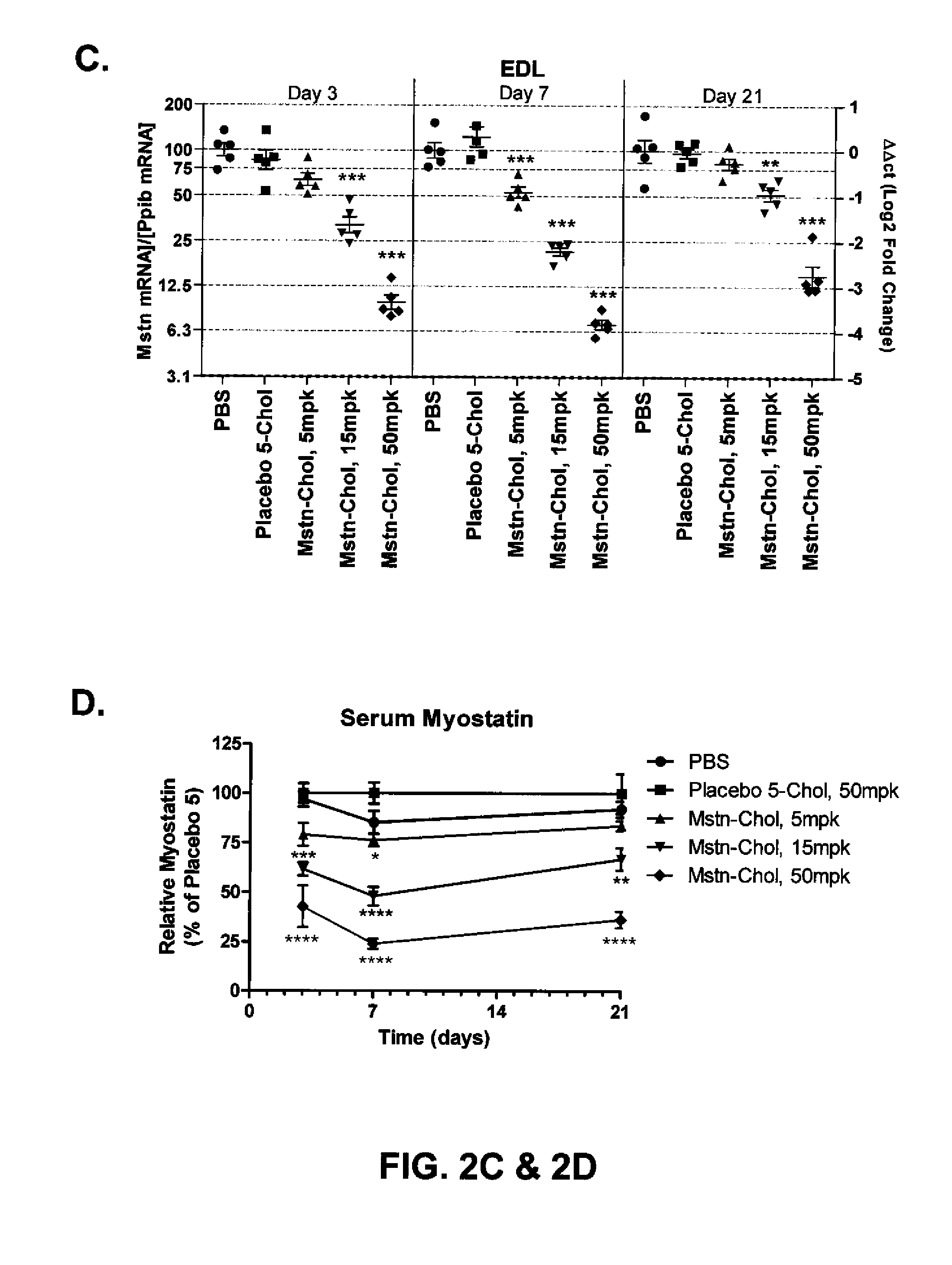
Prolonged Mstn Knockdown Increases Muscle Size and Alters Muscle Fatigue Profile
[0203]Body Composition—
[0204]Animal body composition was measured by quantitative magnetic resonance spectroscopy using an EchoMRI instrument (Echo Medical Systems, Houston, Tex.), in order to determine lean / fat mass. Measurements were made 20 days after initiation of siRNA dosing.
[0205]Micro-CT Imaging and Data Analysis—
[0206]Using the LaTheta micro-CT (LaTheta LCT-100A™, Aloka, Echo Medical Systems, Houston, Tex.), a stack of 10 slices was scanned between the knee and fibula-tibia junction. Images were analyzed as previously described (Weber, H. et al., 2012, J. Appl. Physiol. 112:2087-98) to find the largest cross sectional area (CSA) of whole muscle in the lower leg. Mice were scanned at day-1 with respect to siRNA dosing, and also scanned at day 3, 7, 14 and 21.
[0207]In Situ Muscle Function Assay—
[0208]A custom build in situ assay system, as previously described (see Weber, H. et al., supra), was pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com