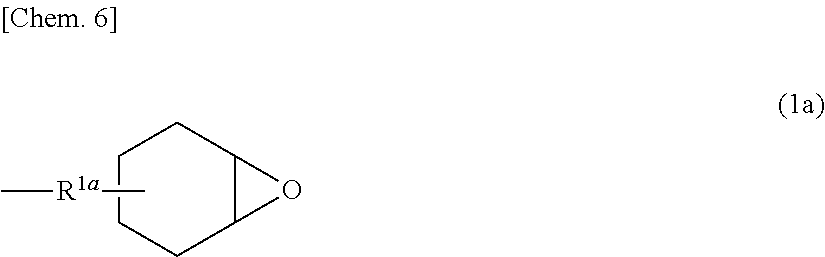Polyorganosilsesquioxane, hard coat film, adhesive sheet, and laminate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0192]Materials used were 161.5 mmol (39.79 g) of 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane (hereinafter also referred to as “EMS”), 9 mmol (1.69 g) of phenyltrimethoxysilane (hereinafter also referred to as “PMS”), and 165.9 g of acetone. In a nitrogen stream, the materials were placed in a 300-ml flask (reactor) equipped with a thermometer, a stirrer, a reflux condenser, and a nitrogen inlet tube, followed by temperature rise to 50° C. to give a mixture. To the prepared mixture, was added dropwise 4.70 g (1.7 mmol in terms of potassium carbonate) of a 5% aqueous solution of potassium carbonate over 5 minutes, followed by 1700 mmol (30.60 g) of water added dropwise over 20 minutes. No significant temperature rise occurred during the dropwise additions. The mixture was then subjected to a polycondensation reaction in a nitrogen stream for 4 hours while keeping the temperature at 50° C.
[0193]A product in the reaction mixture after the polycondensation reaction was analyzed and fou...
example 7
[0197]A solution mixture was prepared as a hard-coating composition (curable composition) by blending 100 parts by weight of the epoxy-containing polyorganosilsesquioxane (S-1) prepared in Example 1, 20 parts by weight of methyl isobutyl ketone (supplied by Kanto Chemical Co., Inc.), and 1 part by weight of a curing catalyst 1 ([diphenyl[4-(phenylthio)phenyl]sulfonium tris(pentafluoroethyl)trifluorophosphate]).
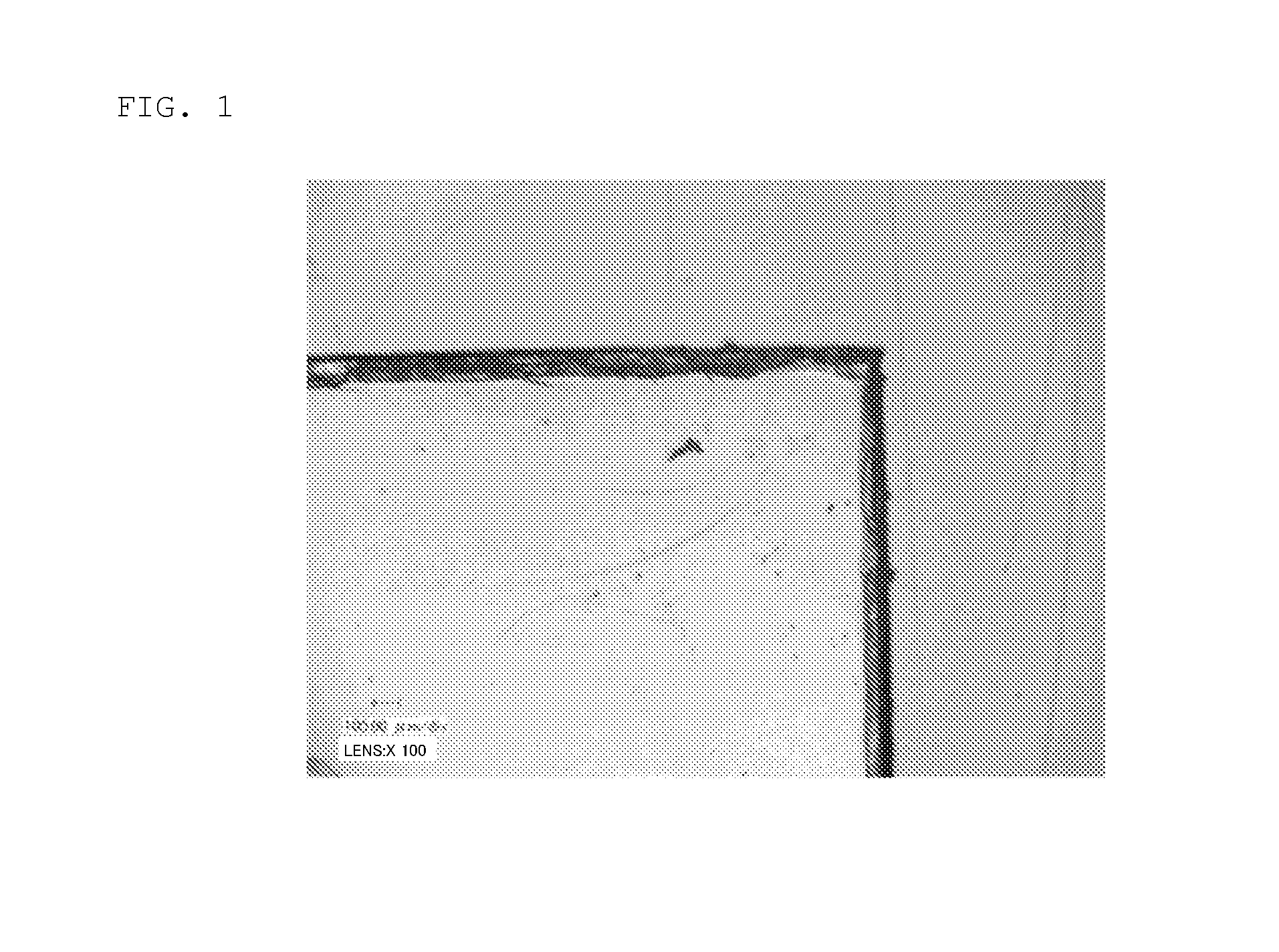
[0198]The above-prepared hard-coating composition was applied onto a PET film (trade name KEB03 W, supplied by Teijin DuPont Films Japan Limited) by flow casting using a wire bar so as to form a hard coat layer having a thickness after curing of 5 μm. The resulting article was left stand in an oven at 70° C. for 10 minutes (for prebaking) and then irradiated with an ultraviolet ray under irradiation conditions at an irradiance of 312 mJ / cm2 and an irradiation intensity of 80 W / cm2. Lastly, the article was subjected to a heat treatment (aging) at 80° C. for 2 hours to cure the ...
examples 8 to 17 and 19
, and Comparative Examples 3 and 4
[0199]Hard-coating compositions were prepared each by a procedure similar to that in Example 7, except for changing the formulation of the hard-coating composition (curable composition) and the thickness of the hard coat layer, as given in Table 2. Hard coat films were prepared each by a procedure similar to that in Example 7, except for using the prepared corresponding hard-coating composition and changing the thickness of the hard coat layer as given in Table 2. In Table 2, the blending amounts of starting materials for the curable compositions are indicated in part by weight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com