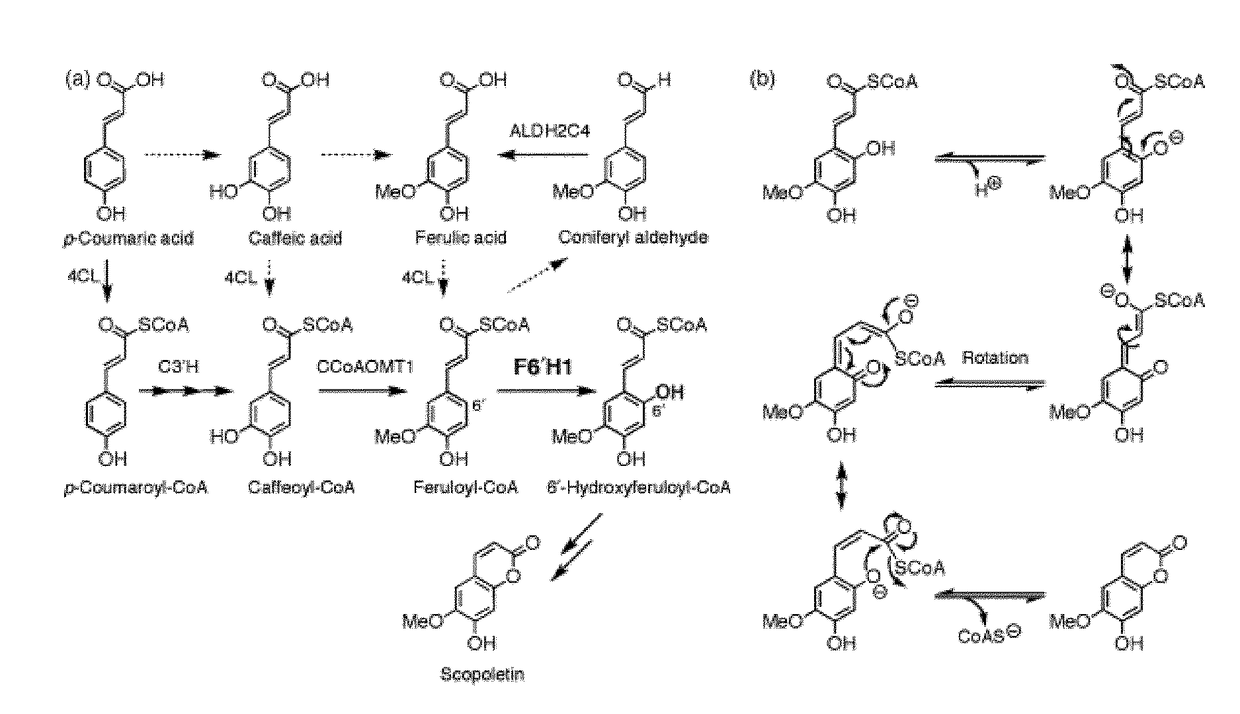
Method of increasing resistance against soybean rust in transgenic plants by increasing the scopoletin content
a technology of scopoletin and soybean rust, which is applied in the direction of plant peptides, biochemistry apparatus and processes, enzymes, etc., can solve the problems of host serious hampered in development or die-off, high susceptibility to epidemic-like spreading of diseases, and high yield reduction, so as to increase the resistance against fungal pathogens, increase the content of scopoletin, and increase the expression of f6h1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ethods
[0323]The chemical synthesis of oligonucleotides can be affected, for example, in the known fashion using the phosphoamidite method (Voet, Voet, 2nd Edition, Wiley Press New York, pages 896-897). The cloning steps carried out for the purposes of the present invention such as, for example, restriction cleavages, agarose gel electrophoresis, purification of DNA fragments, transfer of nucleic acids to nitrocellulose and nylon membranes, linking DNA fragments, transformation of E. coli cells, bacterial cultures, phage multiplication and sequence analysis of recombinant DNA, are carried out as described by Sambrook et al. Cold Spring Harbor Laboratory Press (1989), ISBN 0-87969-309-6. The sequencing of recombinant DNA molecules is carried out with an MWG-Licor laser fluorescence DNA sequencer following the method of Sanger (Sanger et al., Proc. Natl. Acad. Sci. USA 74, 5463 (1977).
Example 2: Cloning of Overexpression Vector Constructs for Transient N. benthamiana Transformation
[032...
example 4
ng Abundance of Gene Transcripts
[0335]Total RNA was extracted from leaves of the described Arabidopsis mutants as described by Chomczynski and Sacchi (1987). 1 μg RNA was transcribed to cDNA using random primers (9-mers) and RevertAid™ reverse transcriptase (Fermentas) according to manufacturer's instructions. Accumulation of gene transcripts was quantified in an ABI7300 using SYBR green (Invitrogen) at the following conditions for RT-qPCR: 50° C. for 2 min, 95° C. for 10 min, 95° C. for 15 s, 60° C. for 1 min, 95° C. for 15 s, 60° C. for 1 min, and 95° C. for 15 s (the third and fourth steps were repeated 40 times).
Primers specifically hybridizing to F6H1 gene (SEQ ID No 1):F6H1_RT_F:(SEQ ID NO: 81)5′-CTCAGCCTCTTCTTTGTCTC-3F6H1_RT_R:(SEQ ID NO: 82)5′-AAGCCTCCTCACCATCTTC-3′Primers specifically hybridizing to CCoAOMT1 (SEQ ID No 3):CCoAOMT1_RT_F:(SEQ ID NO: 83)5′-ATGGCGACGACAACAACAGAAGC-3CCoAOMT1_RT_R:(SEQ ID NO: 84)5′-GCCAATCACTCCTCCAATTTTCACA-3′Primers specifically hybridizing to A...
example 5
Germination Tests
Example 5a Growth Inhibition of Phakopsora pachyrhizi
[0337]Spores of Phakopsora pachyrhizi were resuspended in H2O supplemented with Tween-20 and 10 μM, 100 μM, 500 μM and 1 mM scopoletin. Spores of Phakopsora pachyrhizi resuspended in H2O supplemented with Tween-20 were used as control. All resuspended spores were transferred onto glass slides. After six hours incubation time the ASR spores were germinated and started to form appressoria.
[0338]The germination rate and appressoria formation rate was determined by quantitative microscopic analysis. Spores showing a visible germtube formation but no thickening of the germ tube tip were counted as “germinated”, whereas the presence of a thickened germ tube tip indicated the formation of an appressoria (FIG. 14a).
[0339]Application of scopoletin to ASR spores decreases the germination and appressoria formation in a dose dependent manner. At 1 mM concentration scopoletin completely abolishes spore germination in-vitro.
Ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| injection volume | aaaaa | aaaaa |
| emission wavelength | aaaaa | aaaaa |
| excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com