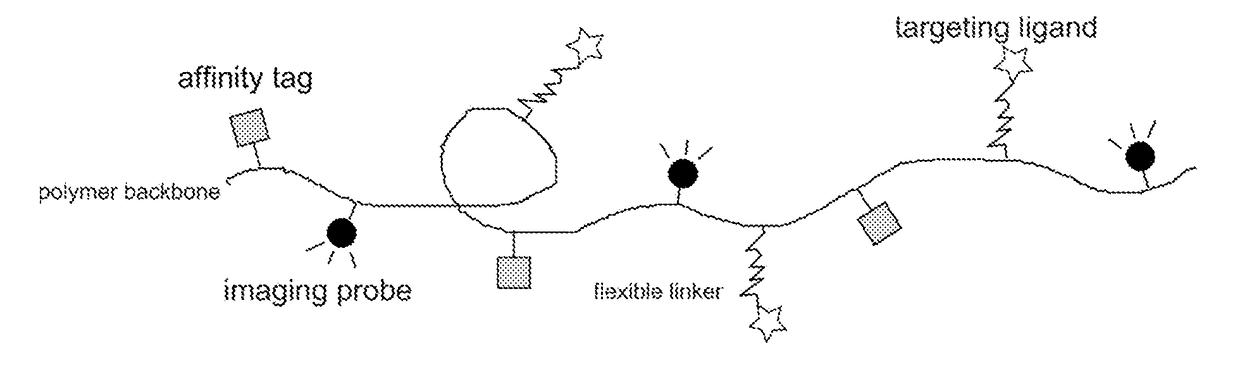
Macromolecular conjugates for visualization and separation of proteins and cells
a technology of macromolecules and conjugates, applied in the field of synthetically prepared macromolecules, can solve the problems of loss of the ability to bind a given antigen, high production cost of antibodies, and easy degradation of antibody molecules, so as to avoid steric hindrance of binding, the effect of inexpensive preparation of polymeric conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
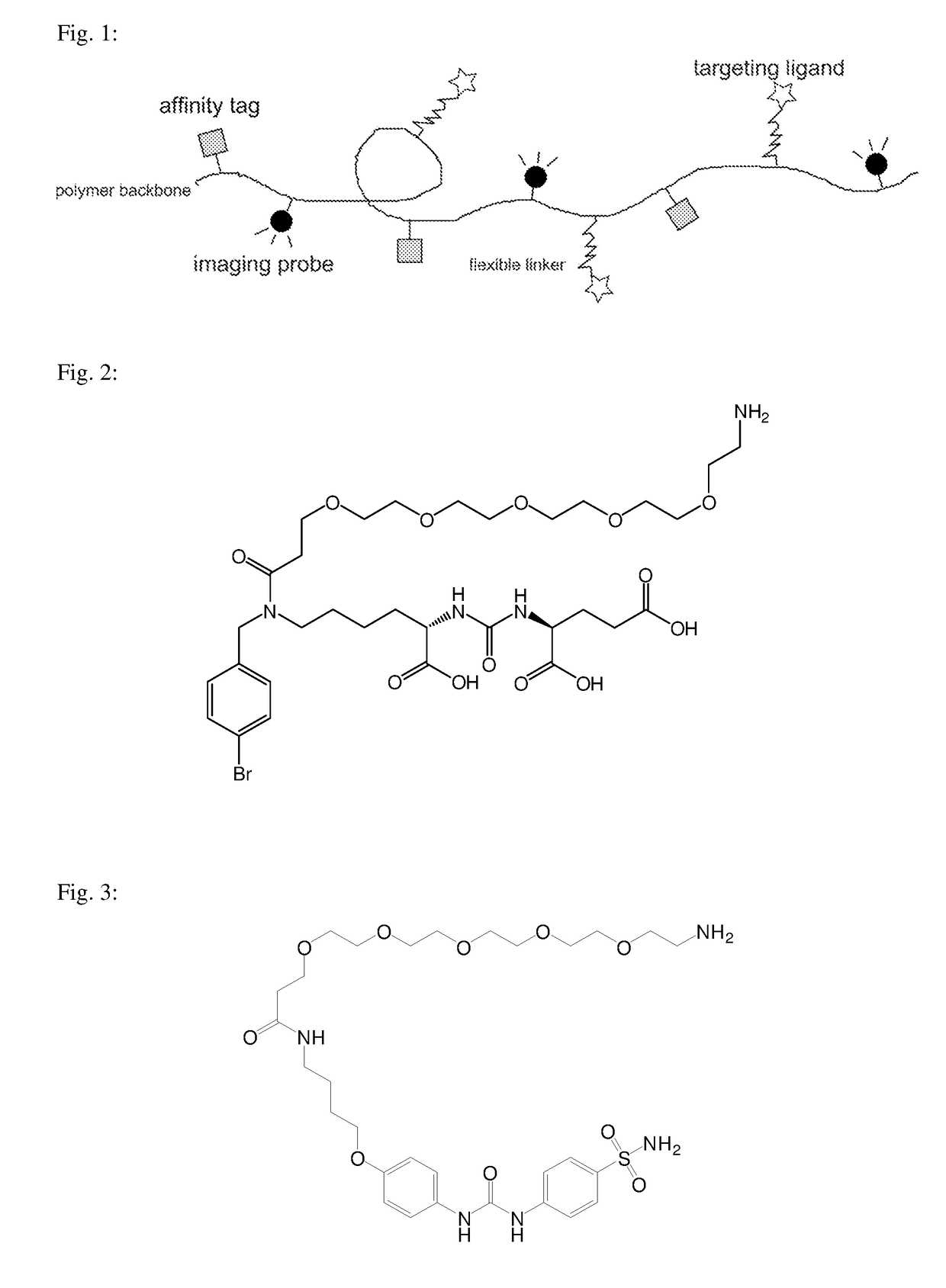
example 1
on of GCPII Inhibitor with a Linker (Compound A)
[0053]
Di-tert-butyl 2-(3-(6-((4-bromobenzyl)amino)-1-(tert-butoxy)-1-oxohexan-2-yl)ureido)pentanedioate; Compound A2: 300 mg (0.615 mmol, 1 eq) di-tert-butyl 2-(3-(6-amino-1-(tert-butoxy)-1-oxohexan-2-yl)ureido)pentanedioate (Compound A1, prepared according to [10]) and 120 mg (0,646 mmol, 1.05 eq) of 4-bromobenzaldehyde was dissolved in 5 ml methanol in a round-bottom flask. 50 μl of glacial acetic acid was added and, after rapid mixing, 120 mg (1.85 mmol, 3.0 eq) of sodium cyanoborohydride in one portion. After 12 hours, the reaction was stopped by adding 10 ml of water. After 10 minutes, the reaction mixture was further diluted with 50 ml of water and was extracted three times with ethyl acetate (3×25 ml). The organic phase was dried and evaporated and the raw product was purified by chromatography on silica gel (eluent: EtOAc+1% ammonia saturated in water, TLC analysis, Rf=0.55). The weight of the obtained pure product was 395 mg (...
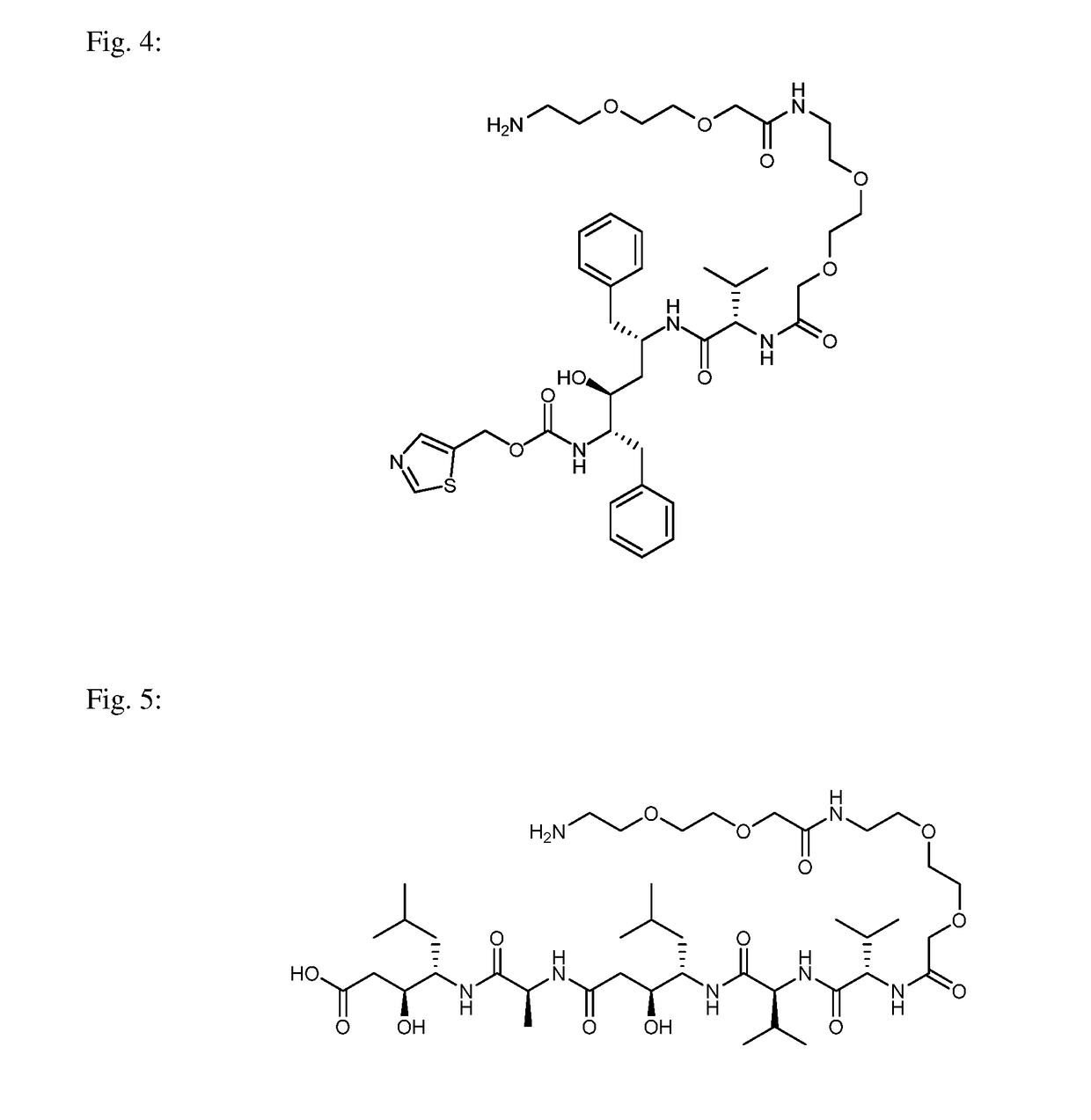
example 2
of Inhibitor of Carbonic Anhydrase IX (Compound B)
[0056]Compound B was prepared according to the scheme below:
methyl 4-(4-((tert-butoxycarbonyl)amino)butoxy)benzoate, Compound B1: 161 mg (1 eq, 1.06 mmol) of methyl 4-hydroxybenzoate, 300 mg (1.5 eq, 1.59 mmol) of tert-butyl (4-hydroxybutyl) carbamate and 400 mg (1.5 eq, 1.59 mmol) of triphenylphosphine was mixed in 10 ml of THF. 312 μl (1.5 eq, 1.59 mmol) of DIAD was then added all at once to the solution and the reaction was stirred overnight. The reaction mixture was then evaporated and the raw product was purified by column chromatography (He:EtOAc 4:1, RF=0.25). The weight of the obtained white powder was 260 mg, representing a 75% yield.
[0057]Note: the methyl 4-hydroxybenzoate had the same RF as the product, so 1.5 eq was used with other compounds.
[0058]MS (ESI+): counted for C17H25O5N [MNa]+ 346.17. Found 346.2. 1H NMR (400 MHz; CDCl3) δ 7.95 (d; J=8.9 Hz; 2H); 6.87 (d; J=8.9 Hz; 2H); 4.71 (s; 1H); 3.99 (t; J=6.2 Hz; 2H); 3.85...
example 3
on of HIV-1 Protease Inhibitor with a Linker (Compound C)
[0062]Compound C, based on a commercially available HIV protease inhibitor drug ritonavir (RTV), was synthesized according to the below depicted scheme:
[0063]Isolation of ritonavir (RTV) from commercially available capsules: RTV is suspended in capsules in an oily mixture of rather non-polar compounds. 50 tablets (100 mg RTV each) were cut open and the oily substance was squeezed out into a round-bottom shaped 2 l flask. 200 ml of hexan was added along with 500 ml of diethyl ether. The resulting suspension was triturated and sonicated for 3 hours until all oil turned into a white precipitate. This precipitate was filtered and again triturated / sonicated in pure diethyl ether, after which the pure RTV was filtered. 3.6 g of RTV was obtained (isolation yield 72%). The purity of RTV was determined by HPLC and was well above 99% (analytical HPLC RT=23.7 min).
Partial hydrolysis of ritonavir (RTV), thiazol-5-ylmethyl ((2S,3S,5S)-5-am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flow rate | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com