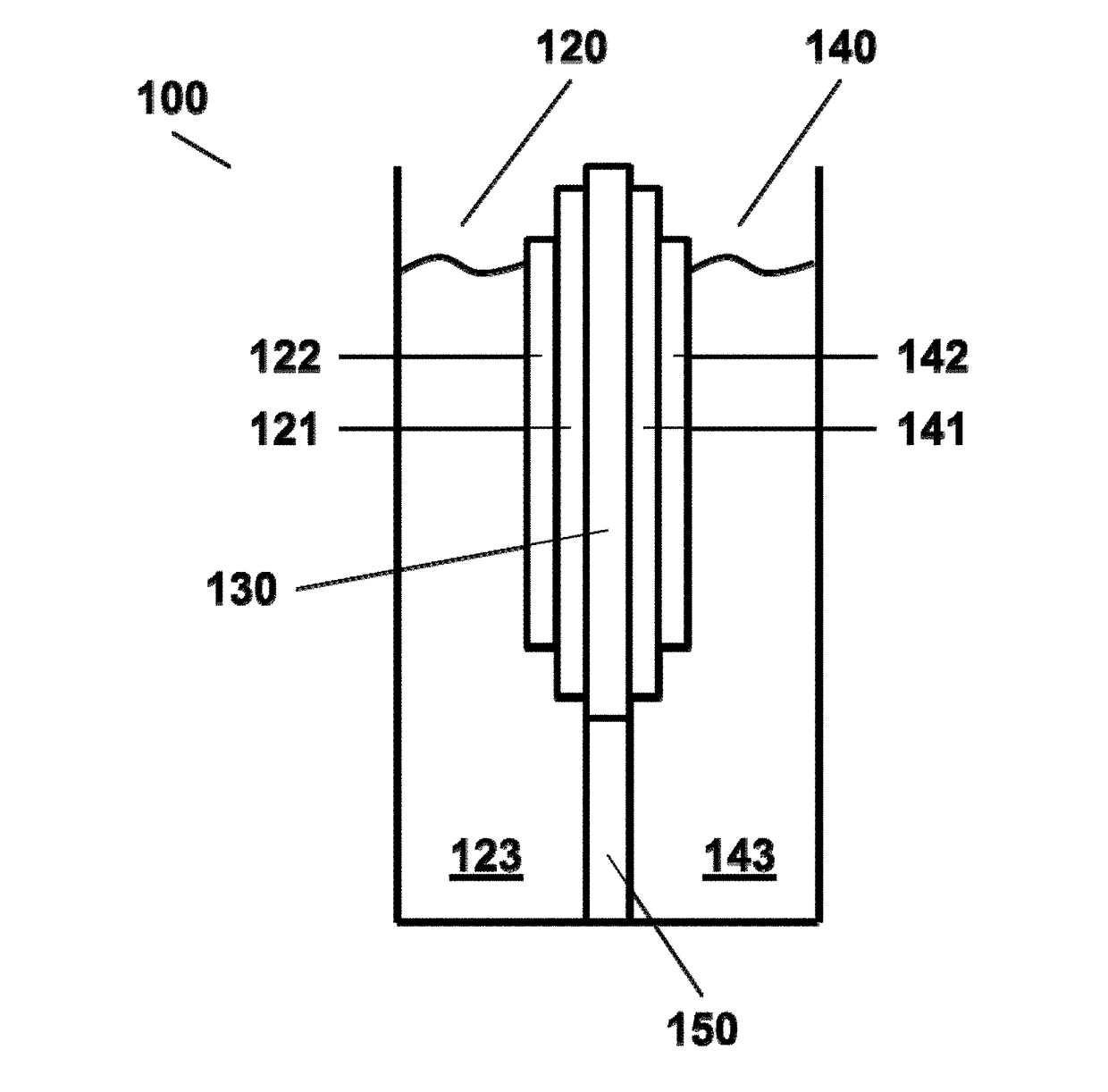
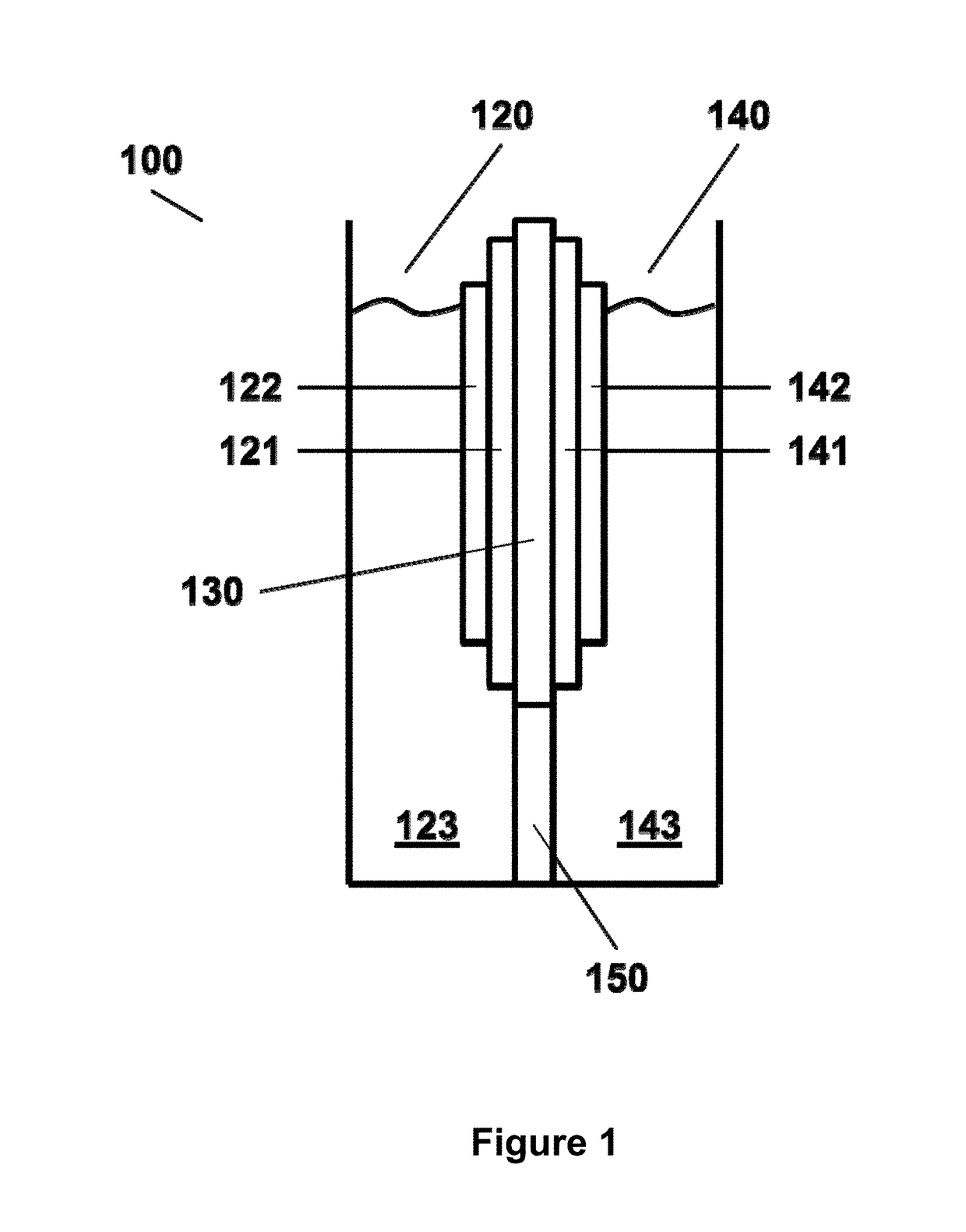
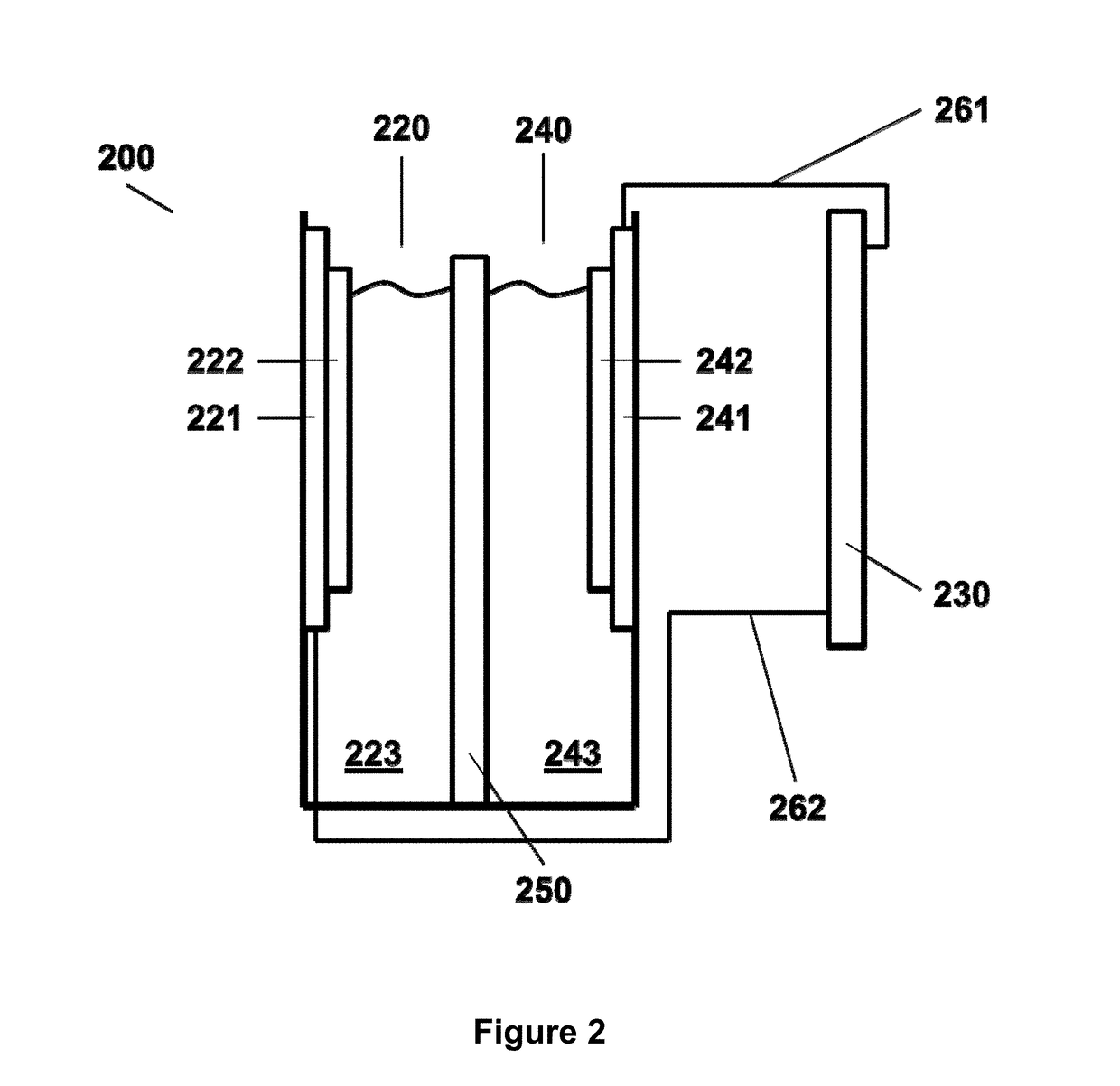
Photoelectrochemical cell for carbon dioxide conversion
a photoelectrochemical and carbon dioxide technology, applied in the field of photoelectrochemical cells, can solve the problems of high overpotential, high overpotential, and largely unpredictable changes in the environmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Transition Metal Dichalcogenide Preparation
[0145]Transition metal dichalcogenides (e.g., MoS2, MoSe2, WS2, and WSe2) were synthesized through direct reaction of pure forms of the relevant elements followed by a vapor transport process in an evacuated ampule at elevated temperatures. In this method, powders of the transition metals and chalcogens (>99.99% trace metal basis purity) were mixed in the desired stoichiometric ratio and loaded into a quartz ampule. The total loaded weight was about one gram. Each quartz ampule had a 1 cm internal diameter and a 20 cm length. The ampule was then evacuated with a turbo molecule pump (−6 mbar) and sealed with a hydrogen torch. The ampule was placed into a two-zone CVD furnace and the temperature of both zones was raised to 1080° C. over 1 day. The temperature of the empty end of the ampule (the cold zone) was then gradually cooled to 950° C. over 4 days, while the other end was maintained at 1080° C., providing single crystalline grains with ...
example 2
Transition Metal Dichalcogenide Nanoflake Preparation
[0146]The crystalline grains produced according to Example 1 were ground to a powder. Nanoflakes were formed by sonicating a dispersion of 300 mg of ground transition metal dichalcogenide powder in 60 mL of isopropanol. The dispersion was sonicated for 30 hours, using a sonication probe (Vibra Cell Sonics 130 W). The sonicated dispersions were then centrifuged for 60 minutes at 2000 rpm, after which the supernatant (the top two thirds of the centrifuged dispersion) was collected by pipette and stored in a glass vial. FIG. 4 shows nanoflakes dispersed in isopropanol, after centrifugation.
example 3
Nanoflake Characterization
[0147]Dynamic light scattering (DLS) experiments were performed to measure nanoflake sizes using a NiComp ZLS 380 system configured with a 35 mW semiconductor laser (670 nm emission) and a thermoelectric temperature control for samples (held at 25° C.). Nanoflakes dispersed in isopropanol were measured, providing the normal distributions shown in FIG. 5.
[0148]The nanoflakes were also characterized by Raman spectroscopy, using a HORIBA LabRAM HR Evolution confocal Raman microscope configured with a 532 nm laser source, a 1200 g / mm grating, a Horiba Andor detector, and a 100x objective. Laser powers at the sample were held between 1-15 mW. Integration times and averaging parameters were chosen to maximize the signal-to-noise ratio. Results are shown in FIG. 6.
[0149]Finally, WSe2 nanoflakes were imaged with scanning electron microscopy (SEM) to understand the microscale morphology of the nanoflakes. Samples were imaged with a Carl Zeiss SEM instrument integrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com