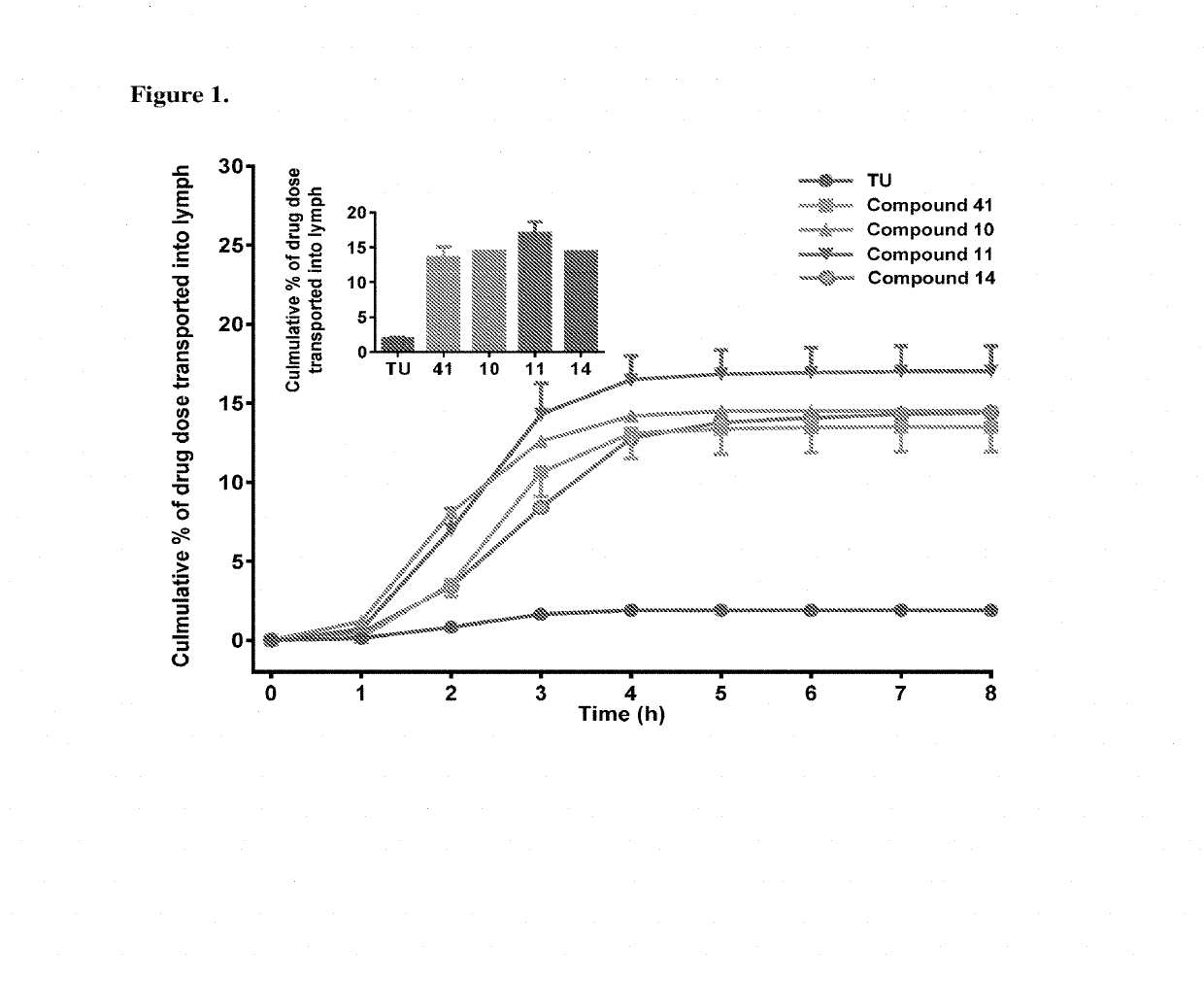
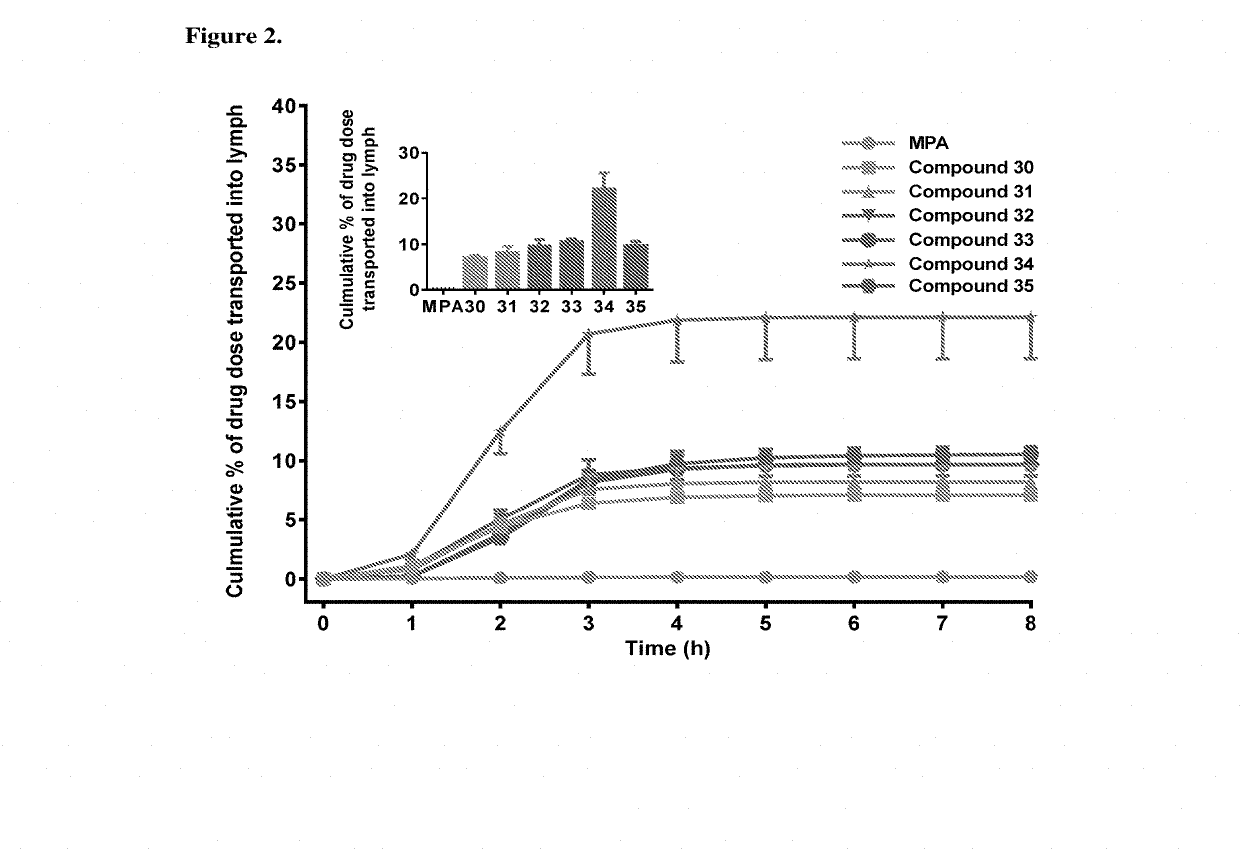
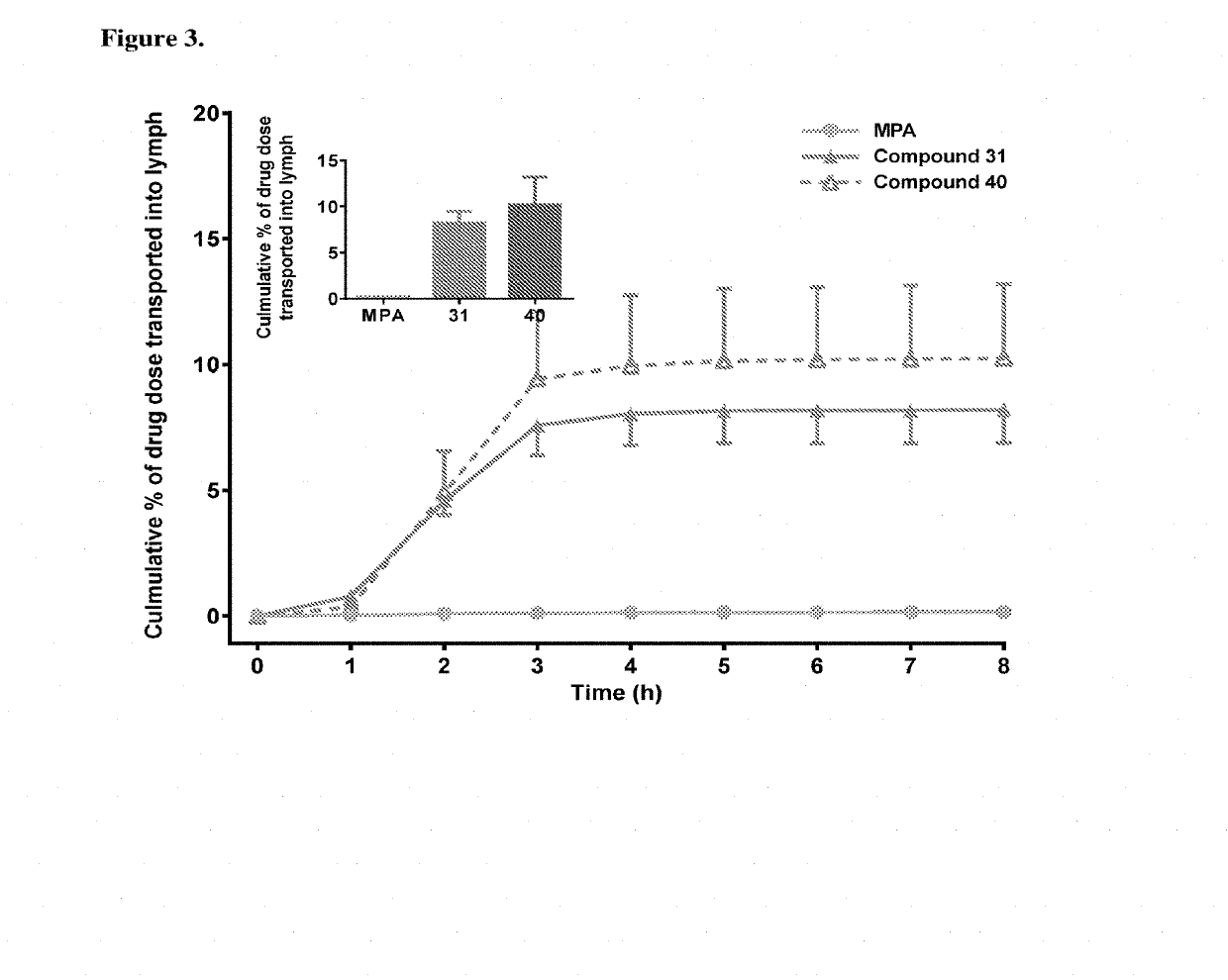
Lymph directing prodrugs
a prodrug and lymph node technology, applied in the field of compounds, can solve the problems of increased toxicity, significant lipophilicity correlation, and reduced potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
or Preparing Compounds of the Formula (I) Wherein Y Represents an Unsubstituted Alkyl Group and L Represents X′ where X′ is O
a) 5-((1,3-Bis(palmitoyloxy)propan-2-yl)oxy)-5-oxopentanoic acid (IV)
[0141]
[0142]4-(Dimethylamino)pyridine (64.4 mg, 0.527 mmol) was added to a solution of diglyceride III (300 mg, 0.527 mmol) and glutaric anhydride I (120 mg, 1.05 mmol) in pyridine / THF / CH2Cl2 (1.5 mL each) and the mixture stirred at rt for two days. The reaction was diluted with ethyl acetate (20 mL), washed with 1 M HCl and brine (20 mL each), dried (MgSO4) and concentrated under reduced pressure to give the crude product. Silica gel chromatography (10% to 15% ethyl acetate / hexanes) gave acid triglyceride IV (140 mg, 39%) as a colourless solid.
[0143]1H NMR (400 MHz, CDCl3) δ 5.26 (m, 1H), 4.31 (dd, J=11.9, 4.3 Hz, 2H), 4.14 (dd, J=11.9, 5.9 Hz, 2H), 2.44 (t, J=7.4 Hz, 2H), 2.42 (t, J=7.4 Hz, 2H), 2.31 (t, J=7.6 Hz, 4H), 1.96 (pent, J=7.3 Hz, 2H), 1.67-1.54 (m, 4H), 1.49-1.18 (m, 48H), 0.88 (...
example 2
or Preparing Compounds of the Formula (I) Wherein Y Represents an α-Methyl Substituted Alkyl Group and L Represents X′ where X′ is O
h) (E)-Methyl 10-(benzyloxy)-2-methyldec-2-enoate (VIII)
[0169]
[0170]Pyridinium chlorochromate (PCC, 39.7 mg, 0.184 mmol) and Celite (30 mg) were added to a solution of alcohol VI (29.0 mg, 0.123 mmol) in CH2Cl2 (1.5 mL) and the reaction stirred at rt for 1.5 hours. The resulting dark suspension was filtered through a short pad of silica gel, eluting with 50% ethyl acetate / hexanes, and the eluent concentrated under reduced pressure to give the crude aldehyde, which was immediately re-dissolved in toluene (1.5 mL). Ylide VII (85.5 mg, 0.245 mmol) was added and the mixture heated at reflux for 20 hours. The reaction was cooled to rt and concentrated under reduced pressure to give the crude product. Purification by silica gel chromatography (5% to 8% ethyl acetate / hexanes) gave α,β-unsaturated methyl ester VIII (26.2 mg, 70%) as a yellow oil.
[0171]1H NMR (4...
example 3
or Preparing Compounds of the Formula (I) Wherein Y Represents a β-Methyl Substituted Alkyl Group and L Represents X′ where X′ is O
n) (7-(Benzyloxy)hept-1-yn-1-yl)trimethylsilane (XIV)
[0187]
[0188]n-Butyllithium (n-BuLi, 1.6 M in hexanes, 765 μL, 1.23 mmol) was added slowly to a solution of TMS-acetylene (198 μL, 1.40 mmol) in THF (1.5 mL) at −78° C. and the mixture stirred at −78° C. for five minutes then warmed to rt and stirred for a further 15 minutes. The reaction was re-cooled to −50° C., a solution of bromide XIII (90.0 mg, 0.350 mmol) in THF (1 mL) was added drop wise and the mixture stirred at −50° C. for 15 minutes and then at rt for 17 hours. The reaction was diluted with brine (15 mL) and the aqueous phase extracted with ethyl acetate (3×15 mL). The combined organic extracts were washed with brine (30 mL), dried (MgSO4) and concentrated under reduced pressure to give the crude product. Purification by silica gel chromatography (4% to 5% ethyl acetate / hexanes) gave TMS alk...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com