Immobilized proteins and use thereof
a technology of immobilized proteins and proteins, applied in the field of immobilized protein materials, can solve the problems of unsuitable other environments, undesired interactions between enzymes and solid supports, deactivation of enzymes, etc., and achieve the effects of reducing catalytic activity, facilitating recycling of immobilized enzyme materials, and speeding up the rate-determining step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Chelating CPG Carriers for Immobilization and Purification of Polyhistidine-Tagged Enzymes
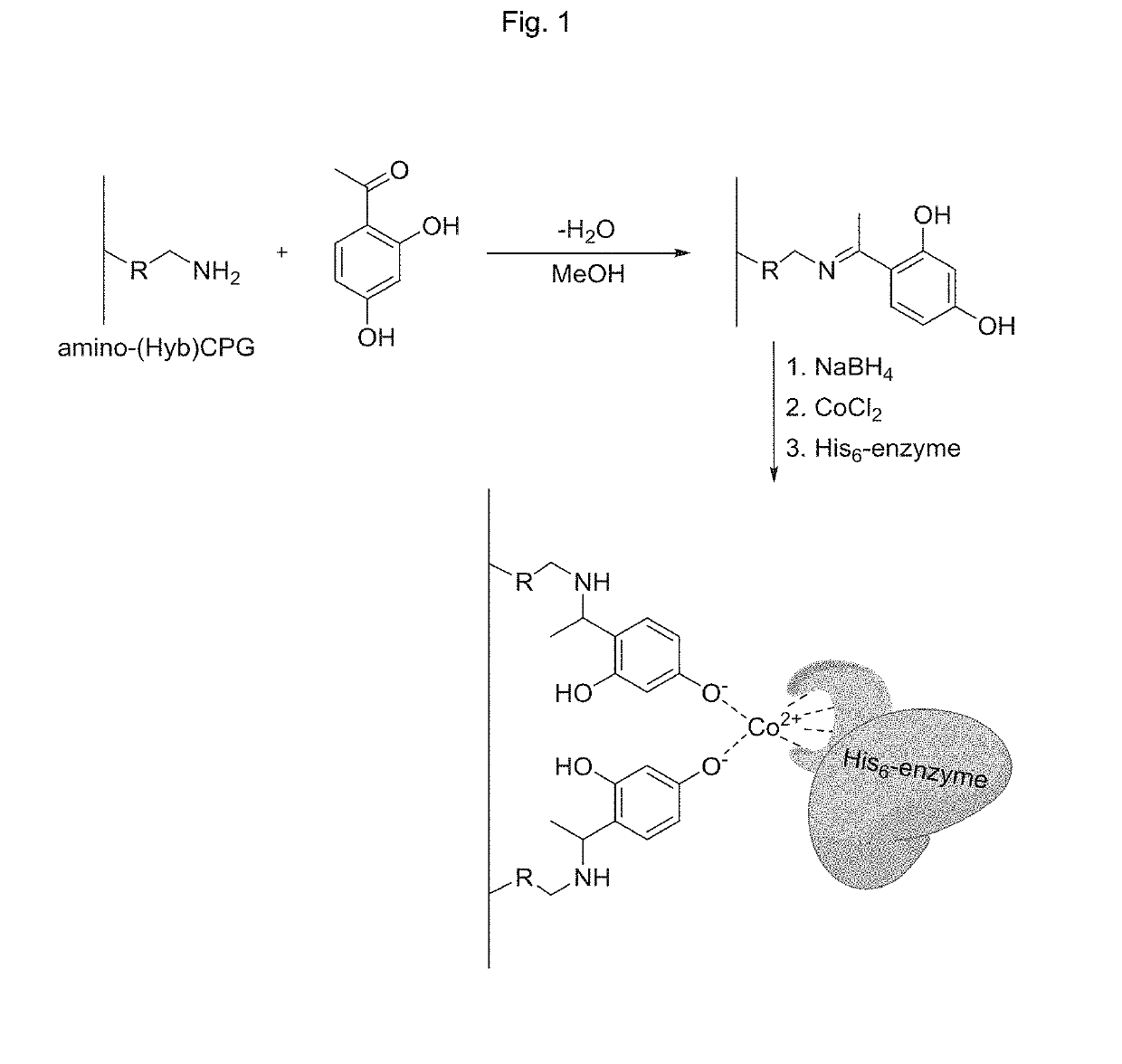
[0122]Amino-CPG (5 g) of desired type was treated with 2,4-dihydroxy acetophenone (1.5 equiv. to the amino functionalities of the CPG) in methanol (200 mL) with continuous stirring for 60 min. The formed imine was reduced by sequential addition of sodium borohydride (4 equiv.) with continuous stirring for 60 min. The solid material was filtrated, rinsed with saturated aqueous sodium carbonate solution, water and then ethanol, and then dried at 80° C. for 2 h. The particles were then immersed in a saturated aqueous solution of CoCl2 (100 mL). After filtration and rinsing with water and ethanol, the particles were dried at 80° C. for 2 h. The properties of the different chelating CPG carriers are shown in Table 1.
TABLE 1Amino Cobalt(II) Chelating CPG Porosity1 derivatization3 loading4 carrier (A) (μmol / g) (μmol / g)LCAA CPG (Co2+) 533 166 2.8 HybCPG VBC (Co2+) 526 2 398 18.7 HybCPG ...
example 2
Preparation of Chelating Porous Polystyrene and Polymethacrylate Carriers for Immobilization and Purification of Polyhistidine-Tagged Enzymes
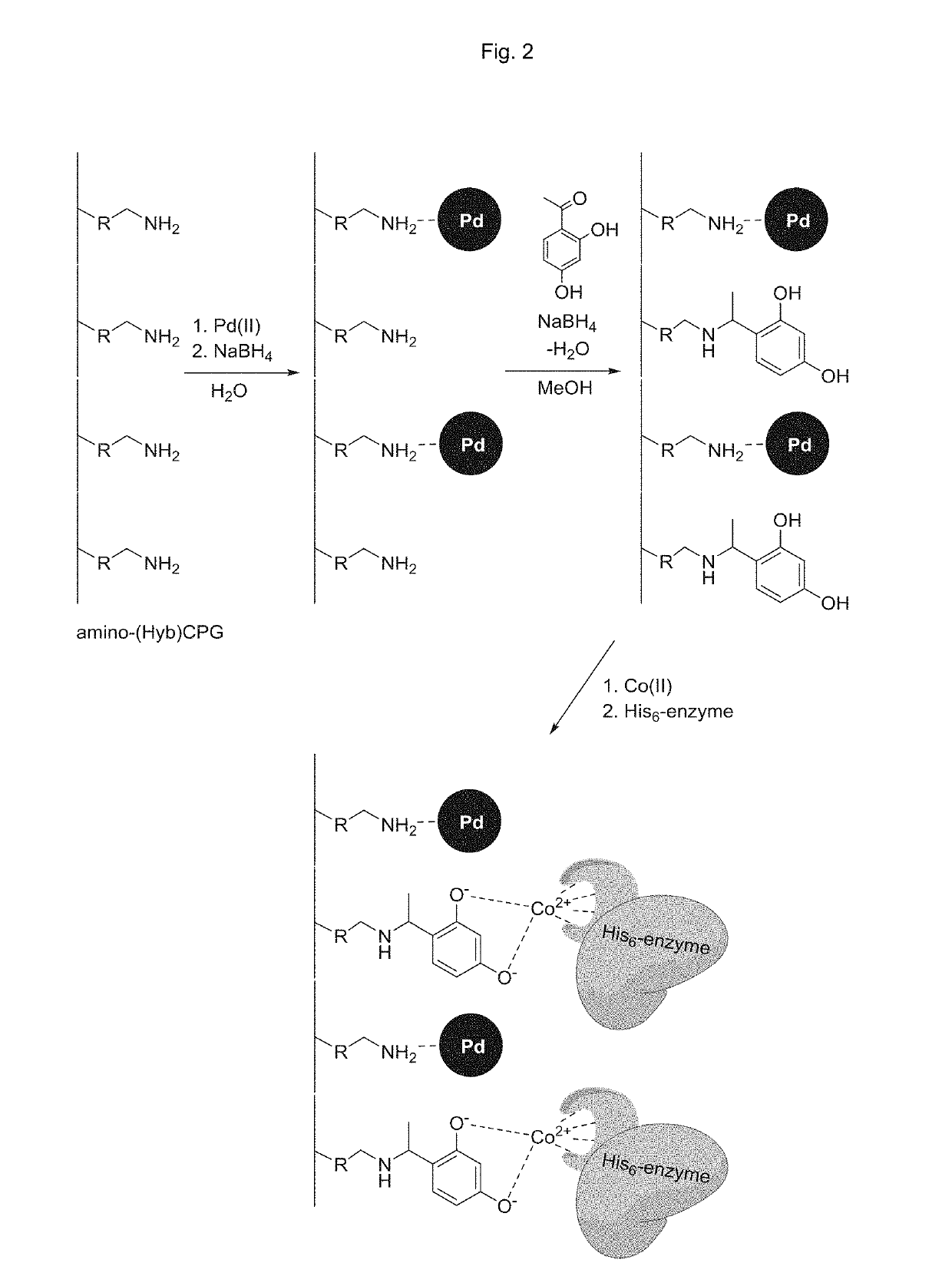
[0124]Washed (water / ethanol 1:1, 400 mL) and dried (vacuum 16 h after filtration) amino functionalized porous organic polymer particles (2 g) of desired type (see below) was treated with 2,4-dihydroxy acetophenone (1.5 equiv. to the amino functionalities of the plastic) in methanol (50 mL) with continuous stirring for 60 min. The formed imine was reduced by sequential addition of sodium borohydride (4 equiv.) with continuous stirring for 60 min. The solid material was filtrated, rinsed with saturated aqueous sodium carbonate solution, water and then ethanol, and then dried in vacuum at 25° C. for 16 h.
[0125]The particles were then immersed in a saturated aqueous solution of CoCl2 (100 mL). After filtration and rinsing with water and ethanol, the particles were dried in vacuum at 25° C. for 16 h. The properties of the different chelating porous ...
example 3
Immobilization of Polyhistidine-Tagged Enzymes on Chelating Carriers
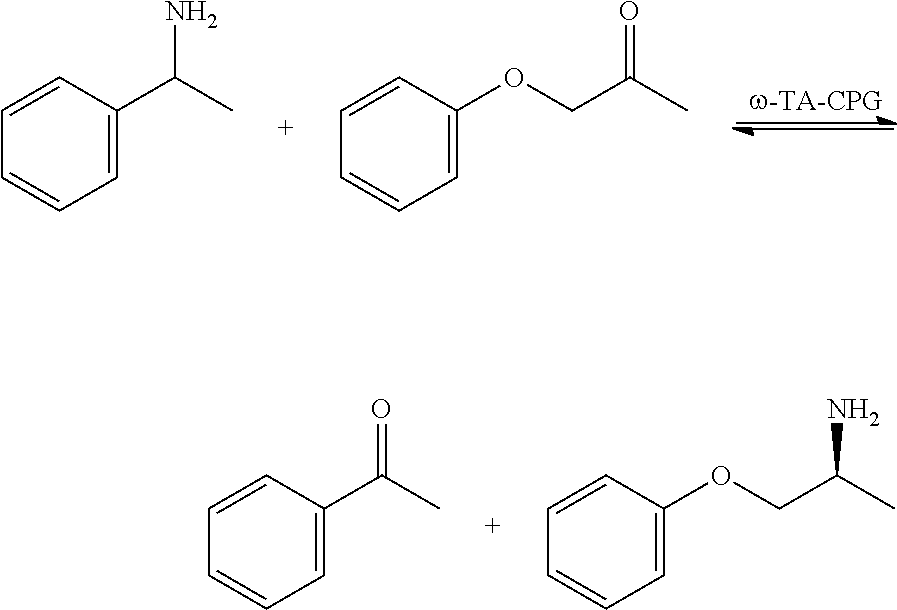
[0126]The cell culture supernatants containing CalA or CalB were used without buffering. The cell lysates of w-TA were prepared by cell resuspension in HEPES buffer (50 mM, 500 mM NaCl, pH 8.3). After addition of detergents (BugBuster™ 10×, Novagen), cell debris was removed by centrifugation. The chelating CPG carrier was immersed in the lysates or supernatants followed by stirring on an orbital shaker (150 rpm). Bradford analyzed samples of the solutions during immobilization confirmed the completion of the binding and saturation of the chelating CPG carrier as the protein concentration seized to decrease. Activity assays were also performed with the solutions after removal of the CPG carrier by filtration. The immobilized preparations were then rinsed with buffer (MOPS (50 mM, pH 7.4) for CalA and CalB; HEPES (see above) for ω-TA) and dried under vacuum for 16 h.
[0127]Extraction of immobilized enzyme from the CPG ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameters | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com