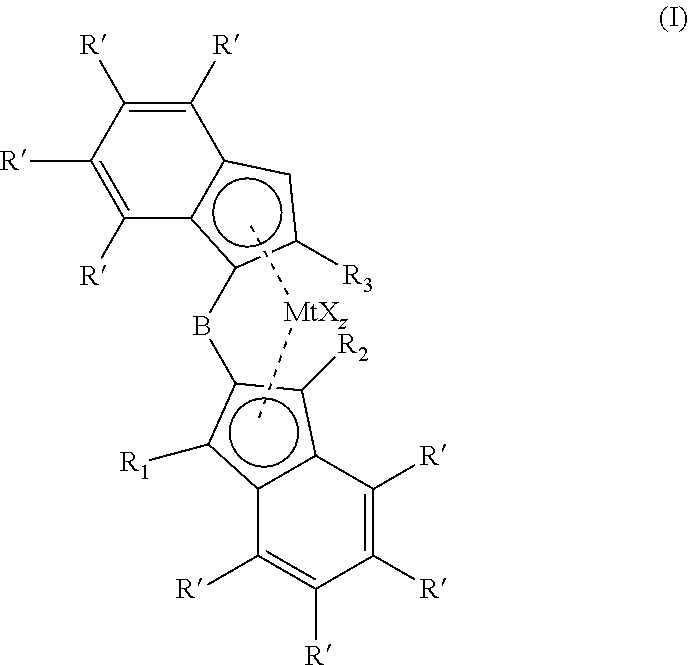
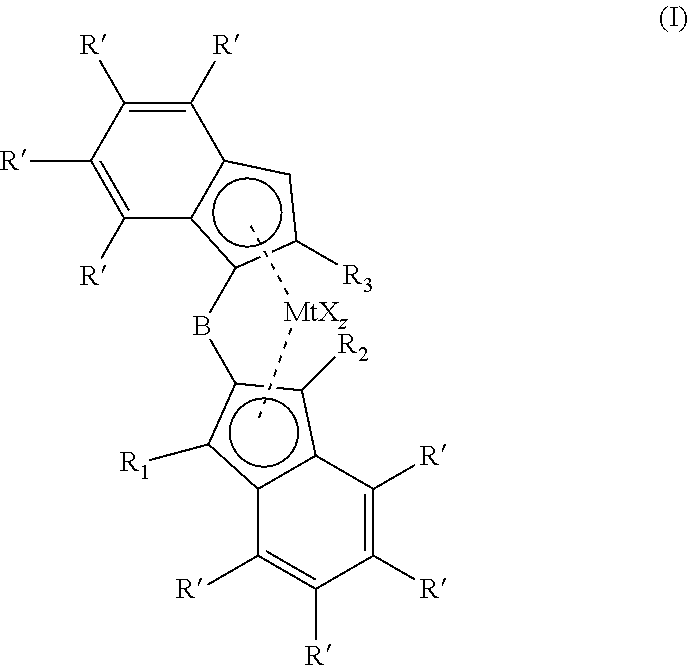
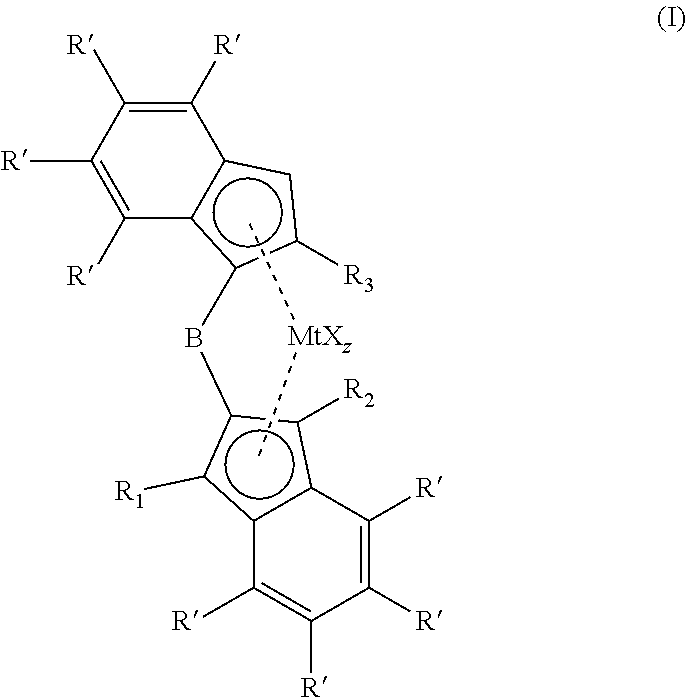
1,2-phenylene bridged 1-indenyl-2-indenyl metallocene complexes for olefin polymerisation
a metallocene complex, 1,2-phenylene technology, applied in the field of substitution 1, 2phenylene bridged 1indenyl 2indenyl metallocene complexes, can solve the problems of low molecular weight of polymers, and achieve high molecular weight, high yield, and high 1-hexene reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
General Considerations
[0063]All manipulations were carried out under an atmosphere of dry, O2-free N2 employing an Innovative Technology glove box and a Schlenk vacuum-line. Tetrahydrofuran (THF), toluene, methylene chloride, hexane and pentane were purified with a Grubbs-type column system manufactured by Innovative Technology and dispensed into thick-walled Schlenk glass flasks equipped with Teflon-valve stopcocks. Pyridine was dried over the appropriate agents and distilled into the same kind of storage flasks. Anhydrous benzene (Alfa, 99.8%, packaged under argon) was purchased and used as received. Deuterated solvents were dried over the appropriate agents, vacuum-transferred into storage flasks with Teflon stopcocks and degassed accordingly (CDCl3, C6D6 and CD2Cl2). 1H, 11B, 13C and 31 P NMR spectra were recorded at 25° C. Bruker 400 MHz spectrometers. Chemical shifts are given relative to SiMe4 and referenced to the residue solvent signal (1H, 13C). 11B and 31P resonances were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| melt mass flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com