Process for preparation of beta -lactams at constantly high concentration of reactants
a technology of reactants and betalactams, which is applied in the field of process for preparing betalactams at constantly high concentration of reactants, can solve the problems of enzymes present that may decompose the starting acylating agent, and the reaction typically involves costly steps, so as to improve the yield of beta-lactam derivatives, improve the molar ratio, and reduce the effect of acylating agent degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Enzymatic Preparation of Amoxicillin from D-HPGM and 6-APA
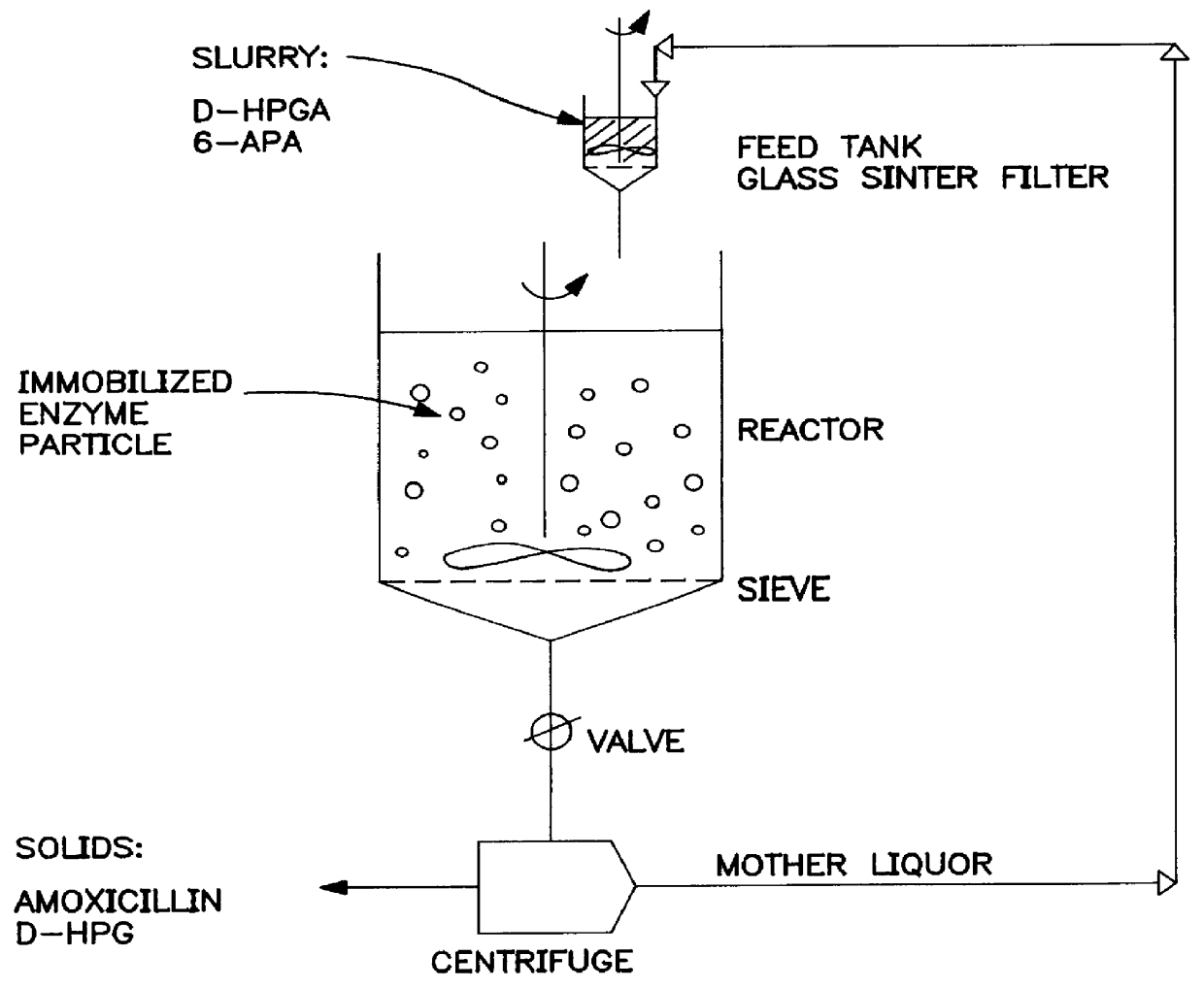
The equipment for this experiment consisted of (see FIG. 1) a thermostated reactor having a volume of 1.5 liters, equipped with a three-bladed impeller and a sieve with slots 180 .mu.m (open area about 32%) The reactor was connected to an autotitrator system using 4 M sulphuric acid as titrand. A valve was positioned at the outlet of the reactor. The outlet of the valve was connected via a pump to a basket centrifuge equipped with a polypropylene bag having a density of 1-5 .mu.m. The outlet from the centrifuge was connected via a pump to a feed tank equipped with a stirrer and a glass sinter bottom. The outlet from the feed tank was connected via a pump to the reactor.
A mixture consisting D-HPGM (36.2 g, 200 mmol) and 6-APA (43.2 g, 200 mmol) in 800 ml water was added to the reactor with the bottom valve closed. The stirring was started. Immobilized Penicillin G acylase (26250 U, size 200-500 .mu.m) made up to 200 ml was add...
example 2
Enzymatic Preparation of Amoxycillin from D-HPGA and 6-APA
The equipment for this experiment is described in example 1.
A mixture consisting of D-HPGA (20.0 g, 120 mmol) and 6-APA in 800 ml water, which was adjusted at a pH value of 6.0 by adding 4 M ammonium hydroxide, was added to the reactor with the bottom valve closed. The stirring was started. Immobilized Penicillin G acylase (26250 U, size 200-500 .mu.m) made up to 200 ml was added to the reactor. The pH value was maintained at 6.0. The reaction temperature was about 20.degree. C. Under these conditions the reaction mixture was almost saturated with D-HPGA and 6-APA. Then the bottom valve was opened allowing the reaction mixture from the reactor to enter the centrifuge. Thereafter, the mother liquor from the centrifuge was pumped into the feed tank wherein D-HPGA (20.0 g, 120 mmol) and 6-APA (20.0 g, 92.5 mmol) were loaded. The total volume of the suspension in the feed tank was kept at about 75 ml. A flow of about 100 ml / min w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com