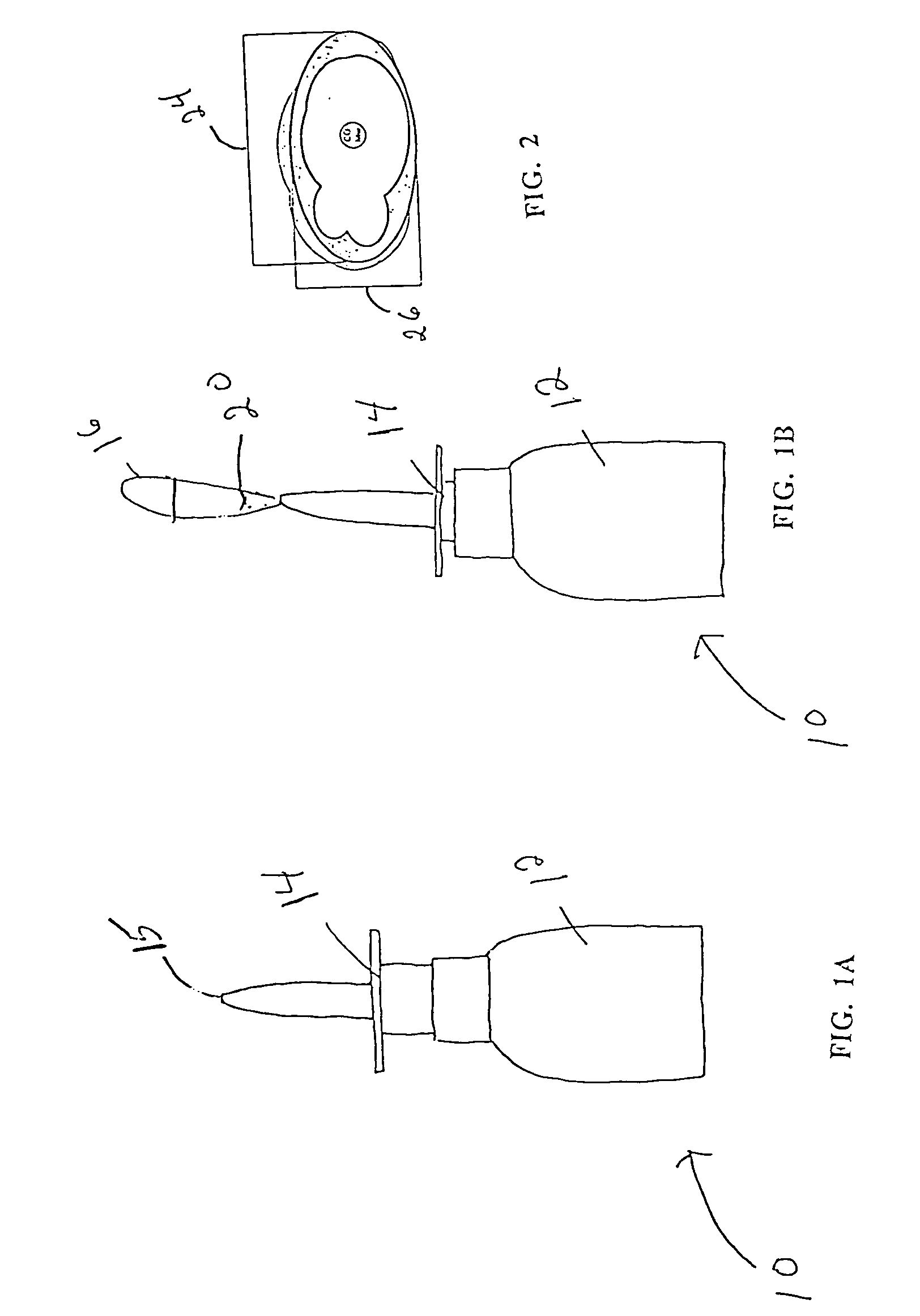
Cyanocobalamin low viscosity aqueous formulations for intranasal delivery
a technology of cyanocobalamin and intranasal delivery, which is applied in the field of cyanocobalamin low viscosity aqueous formulations for intranasal delivery, can solve the problems of affecting affecting the synthesis of dna in any cell in which chromosomal replication is active, and affecting the absorption effect, so as to prevent irritation, increase the shelf life of compositions, and inhibit mucous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparison of Intranasal Cyanocobalamin Solution of the Present Invention with NASCOBAL® and Intramuscular Injections of Cyanocobalamin
[0066]Introduction
[0067]Nascobal® (Cyanocobalamin, USP) is a synthetic form of vitamin B12 with equivalent vitamin B12 activity. The chemical name is 5,6-dimethyl-benzimidazolyl cyanocobamide. Currently, Nascobal® (Cyanocobalamin, USP) is marketed as a self-administered nasal gel. The recommended dose of Nascobal® (Cyanocobalamin, USP) in subjects with vitamin B12 malabsorption who are in remission following inject able vitamin B12 therapy is 500-μg administered intranasally once weekly.
Vitamin B12 deficiency has a number of causes, including malabsorption of vitamin B12 resulting from structural or functional damage to the gastrointestinal system and dietary deficiency of vitamin B12.
The purposes of this study are to compare the bioequivalence of vitamin B12 nasal gel versus the nasal spray, and to evaluate the relative bioavailability of three prep...
example 2
[0086]We conducted a non-blinded, single dose, parallel group study to compare the uptake of Vitamin B12 into the cerebrospinal fluid (CSF) after intranasal and intramuscular administration in healthy male and non-pregnant female volunteers. This study compared CSF levels to plasma levels produced by both formulations.
[0087]Thirty-six healthy male and non-pregnant female subjects, age 18 and over, were enrolled in the study. Eighteen subjects received a single intranasal dose of 500 mcg delivered as a 0.1 mL spray and eighteen subjects received a single intramuscular dose of 100 mcg delivered intramuscularly. Each subject visited the clinical site three times in a one-month period. These visits consisted of a screening visit, one dosing visit and a final visit.
[0088]After each dosing, each subject underwent lumbar puncture only once, with the retrieval of a total 4.0 mL of CSF (4 tubes, 1.0 mL per tube). One third of the subjects had a CSF sample collected at 60 minutes post dosing,...
example 3
Production of a Cyanocobalamin Solution
[0099]A 4000 g batch of a cyanocobalamin solution of the present invention, which had a concentration of 500 mcg / 0.1 g of solution.
[0100]
Starting MaterialsI. Formula RecordIngredient NameTheoretical Weight (Grams)Cyanocobalamin, USP20.0Citric Acid, USP (Anhydrous)4.8Sodium Citrate, USP (Dihydrate)12.8Glycerin, USP89.2Benzalkonium Chloride Solution, NE (50%)1.6Purified Water, USP3871.6*
The 3871.6 grams of water was placed in a stainless steel container, which had been placed on a hot plate. The water was heated to about 30° C. and stirred. Into the heated water was added 12.8 g of sodium citrate while the water was being stirred at 300 rpm for 5 minutes. The 4.8 g of citric acid was then added and stirred for 10 minutes. Into this mixture was added 20.0 g of cyanocobalamin and stirred for 30 minutes at 30° C. at 300 rpm. The hot plate was then turned off. The 89.2 g of glycerin was added and stirred for 5 minutes at 300 rpm. Into the cyanocobala...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com