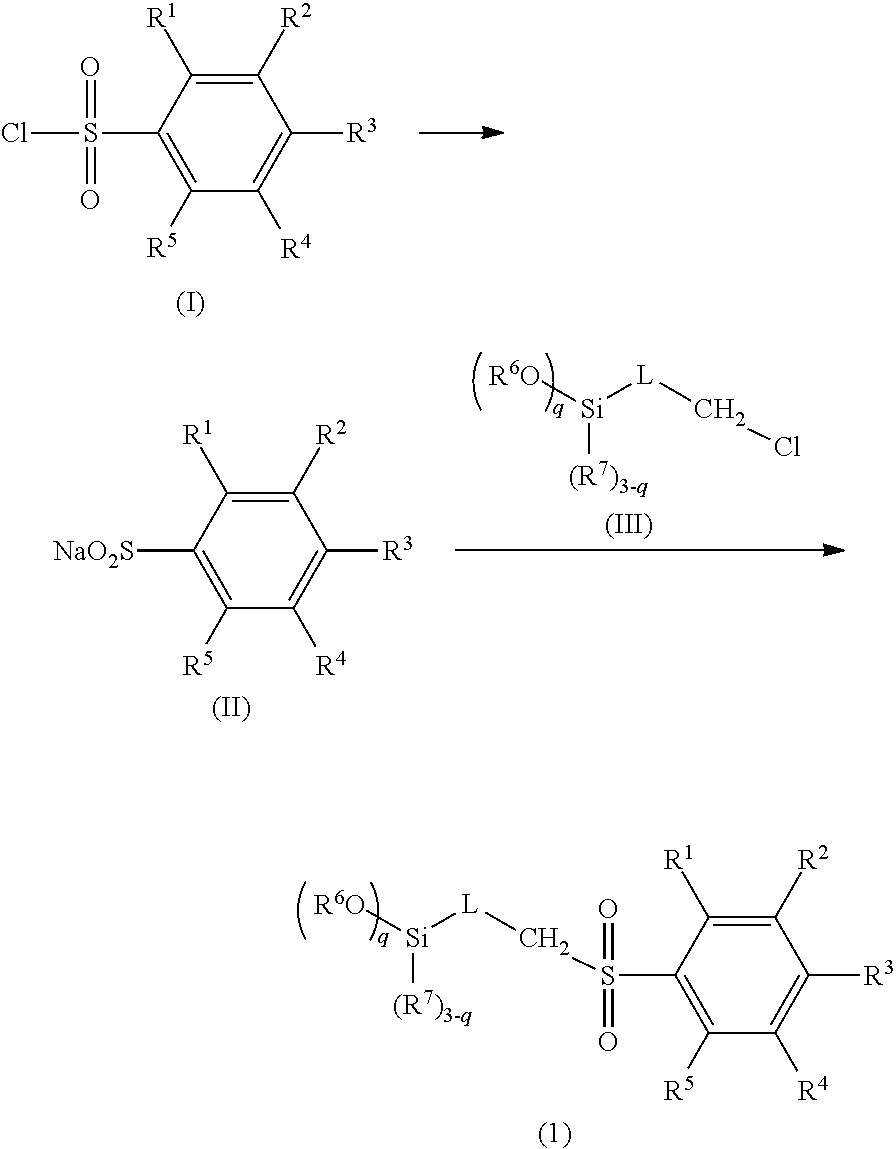
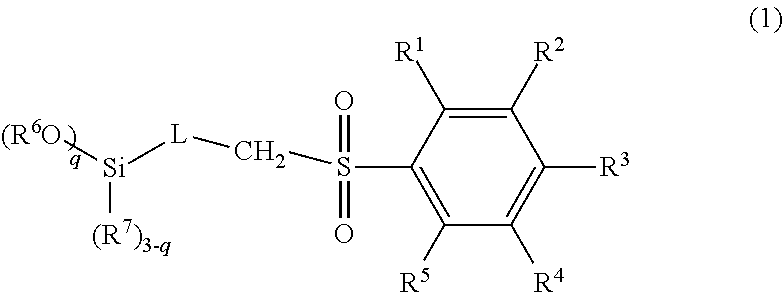
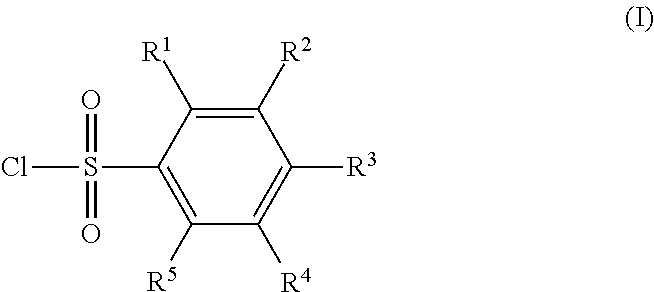
Method for producing silane compound having sulfonyl bond
a technology of sulfonyl bond and silane compound, which is applied in the direction of silicon organic compounds, organic chemistry, chemistry apparatus and processes, etc., can solve the problems of affecting the reflection of active light from the semiconductor substrate, and achieve the suppression of the production of byproducts and side reactions, and the effect of convenient and inexpensive production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Compound 1
[0062]
[0063]32.2 g (0.156 mol) of 4-methoxybenzene-1-sulfonyl chloride, 19.6 g (0.156 mol) of sodium sulfite, 39.3 g (0.467 mol) of sodium bicarbonate, and 100 g of water were placed in a 500-mL recovery flask, and heated to 100° C., and a reaction was caused for 1 hour. Subsequently, 100 g of toluene was added, the mixture was heated until a reflux state, and water was collected by a Dean-Stark apparatus. 25.0 g (0.104 mol) of 3-chloropropyltriethoxysilane, 3.1 g (0.021 mol) of sodium iodide, and 100 g of N-methyl-2-pyrrolidone were further added, and the mixture was stirred under heating for 3 hours while the solvent was distilled off at 150° C. The reaction liquid was separated using toluene and water, activated carbon was added to the organic phase, the organic phase was stirred and filtered, and toluene was removed by an evaporator, to obtain a crude product. The crude product was distilled under reduced pressure, to obtain a compound 1 as a target at a ...
example 2
Production of Compound 2
[0065]
[0066]38.1 g (0.156 mol) of 4-trifluoromethylbenzene-1-sulfonyl chloride, 19.6 g (0.156 mol) of sodium sulfite, 39.3 g (0.467 mol) of sodium bicarbonate, and 100 g of water were placed in a 500-mL recovery flask, and heated to 100° C., and a reaction was caused for 1 hour. Subsequently, 100 g of toluene was added, the mixture was heated until a reflux state, and water was collected by a Dean-Stark apparatus. 25.0 g (0.104 mol) of 3-chloropropyltriethoxysilane, 3.1 g (0.021 mol) of sodium iodide, and 100 g of N-methyl-2-pyrrolidone were further added, and the mixture was stirred under heating for 3 hours while the solvent was distilled off at 150° C. The reaction liquid was separated using toluene and water, activated carbon was added to the organic phase, the organic phase was stirred and filtered, and toluene was removed by an evaporator, to obtain a crude product. The crude product was distilled under reduced pressure, to obtain a compound 2 as a targ...
example 3
Production of Compound 3
[0068]
[0069]24.25 g (0.125 mol) of 4-fluorobenzene-l-sulfonyl chloride, 15.7 g (0.125 mol) of sodium sulfite, 31.4 g (0.374 mol) of sodium bicarbonate, and 100 g of water were placed in a 500-mL recovery flask, and heated to 100° C., and a reaction was caused for 1 hour. Subsequently, 100 g of toluene was added, the mixture was heated until a reflux state, and water was collected by a Dean-Stark apparatus. 20.0 g (0.083 mol) of 3-chloropropyltriethoxysilane, 2.5 g (0.017 mol) of sodium iodide, and 100 g of N-methyl-2-pyrrolidone were further added, and the mixture was stirred under heating for 3 hours while the solvent was distilled off at 150° C. The reaction liquid was separated using toluene and water, activated carbon was added to the organic phase, the organic phase was stirred and filtered, and toluene was removed by an evaporator, to obtain a crude product. The crude product was distilled under reduced pressure, to obtain a compound 3 as a target at a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com