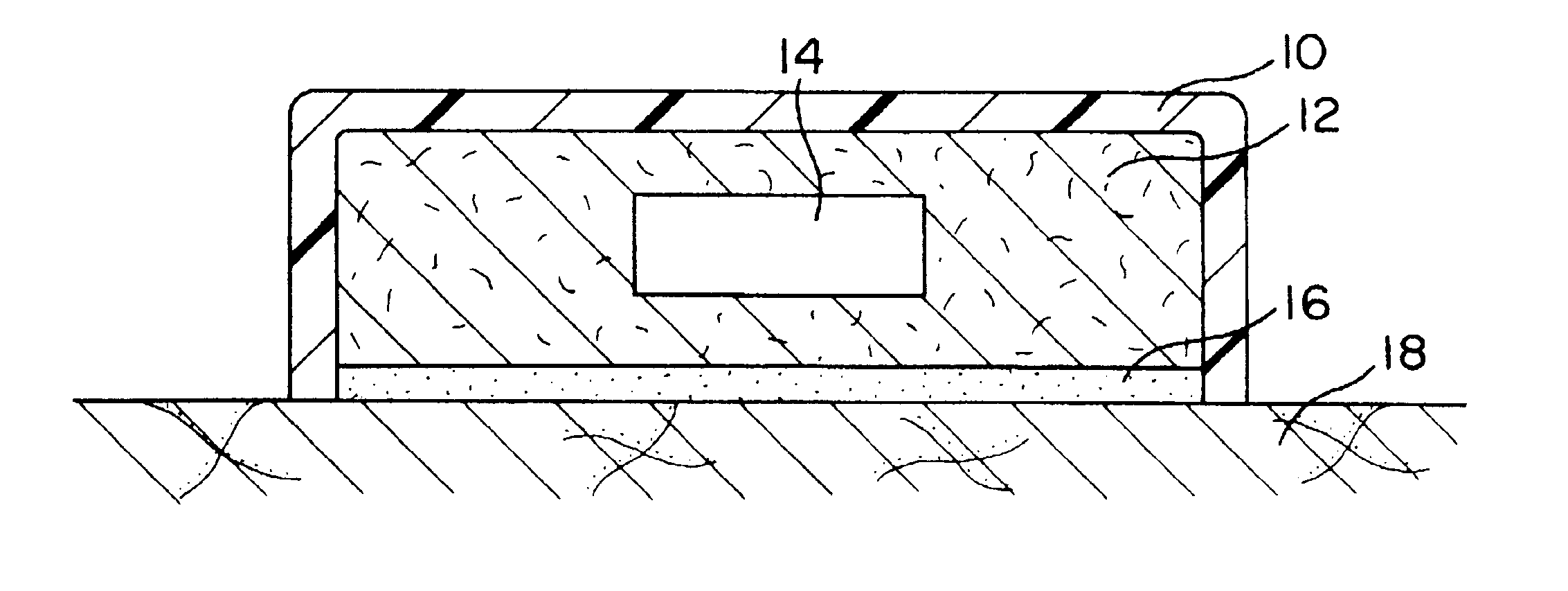
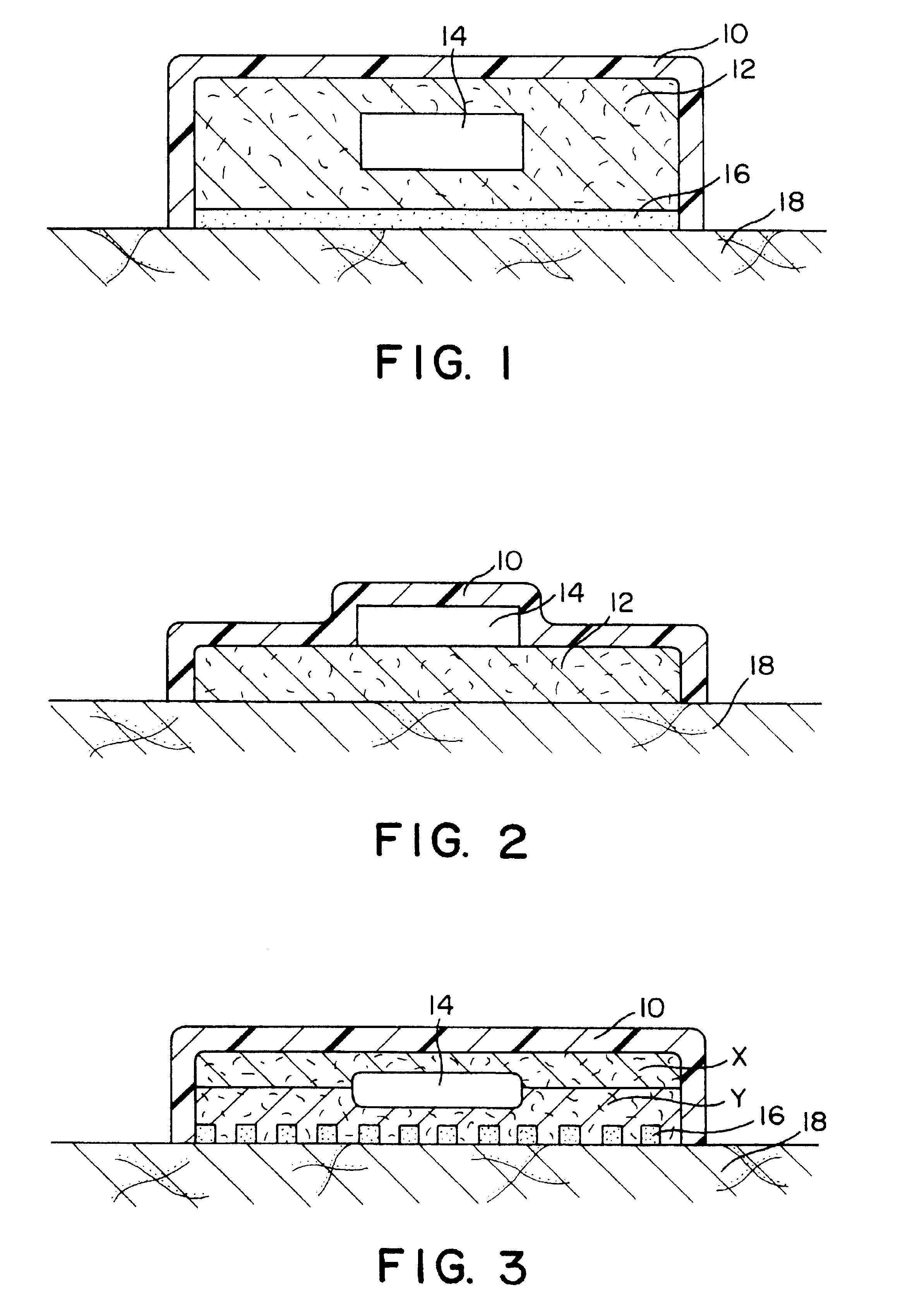
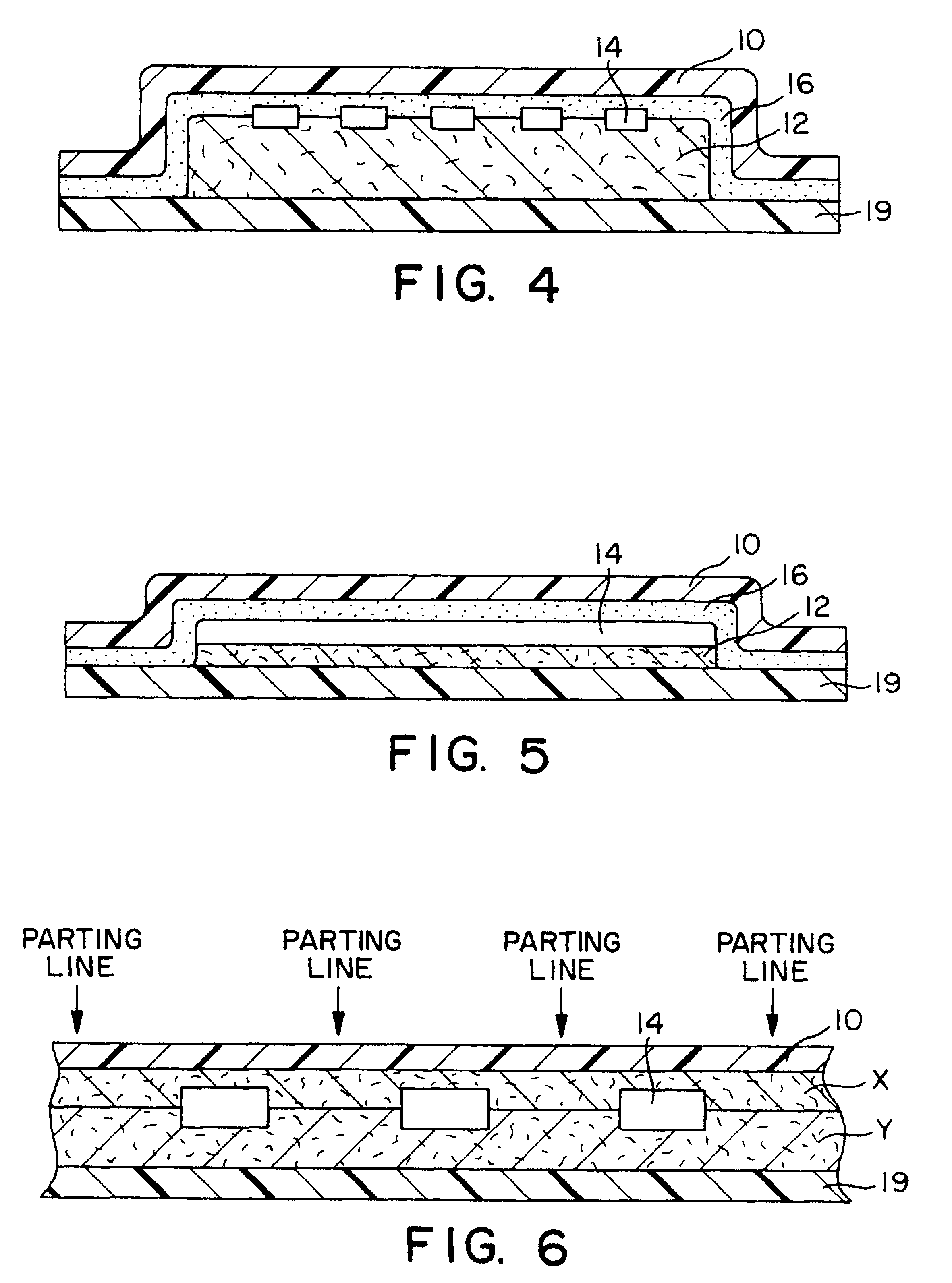
Transdermal therapeutic system
a technology of transdermal therapy and therapeutic system, which is applied in the field of therapeutic system, can solve the problems of thermally sensitive active substances that cannot be used in the system to a limited extent, difficult to manufacture and have a complicated structure, and known systems, so as to facilitate the processing of active substances, increase the permeability of the skin, and simplify the effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
PRODUCTION OF A NICOTINE PLASTER
Nicotine plasters, such as are used to stop people from smoking, are, according to the invention, produced as follows.
A pressure sensitive adhesive material comprising 2.0825 kg of a 40% solution of a self-crosslinking acrylate copolymer, e.g. of 2-ethyl-hexyl acrylate, vinyl acetate, acrylic acid and titanium chelate ester, or DUROTAC 280-2416 of the firm National Starch / Chemical B.V. in a mixture of ethyl acetate, ethanol, hexane and methanol, 147 g of an acrylic resin of dimethylaminoethylmethacrylate and neutral methacrylate (EUDRAGIT E 100 of the firm ROHM PHARMA), and 20 g of a mixed acidic triglyceride of fractionated C.sub.8 -C.sub.10 coconut fatty acids (Miglyol 812 of the firm Dynamit Nobel) are applied to a protective layer vapor-deposited with aluminum on one side and abhesively finished on both sides and the solvent is evaporated at 50.degree. to 80.degree. C. An approximately 300 g / m.sup.2 layer is obtained. From the thus produced pressu...
example 2
PRODUCTION OF A NICOTINE PLASTER
Another nicotine plaster according to the invention may be inventively produced as follows:
A pressure sensitive adhesive material (adhesive 1) comprising 1.9758 kg of a 40% solution of a self-crosslinking acrylate copolymer (DUROTAC 280- 2416 of the firm Delft National & Chemical B.V.) in a mixture of ethyl acetate, ethanol, heptane and methanol, 189.7 g of an acrylic resin of dimethylaminoethylmethacrylate and neutral methacrylate (EUDRAGIT E 100 of the firm ROHM PHARMA), and 20 g of a mixed acidic triglyceride or fractionated C.sub.8 -C.sub.10 coconut fatty acids (Miglyol 812 of the firm Dynamit Nobel) are applied to a protective layer vapor-deposited with aluminum on one side and abhesively finished on both sides and the solvent is evaporated at 50.degree. to 80.degree. C. An approximately 440 g / m.sup.2 layer is obtained. From the thus produced pressure sensitive adhesive layer are punched round discs with a diameter of 51 mm. The projecting edges ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com