Method for defluorinating and degrading complete fluorine substituted compounds
A fluorooctane sulfonate, perfluorooctanoic acid technology, applied in chemical instruments and methods, organic decomposition, solid waste removal, etc., can solve the problems of unclear changes in PFOS, inability to effectively decompose PFOA and PFOS, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the vacuum ultraviolet ray reduction decomposition of perfluorooctanoic acid (PFOA)
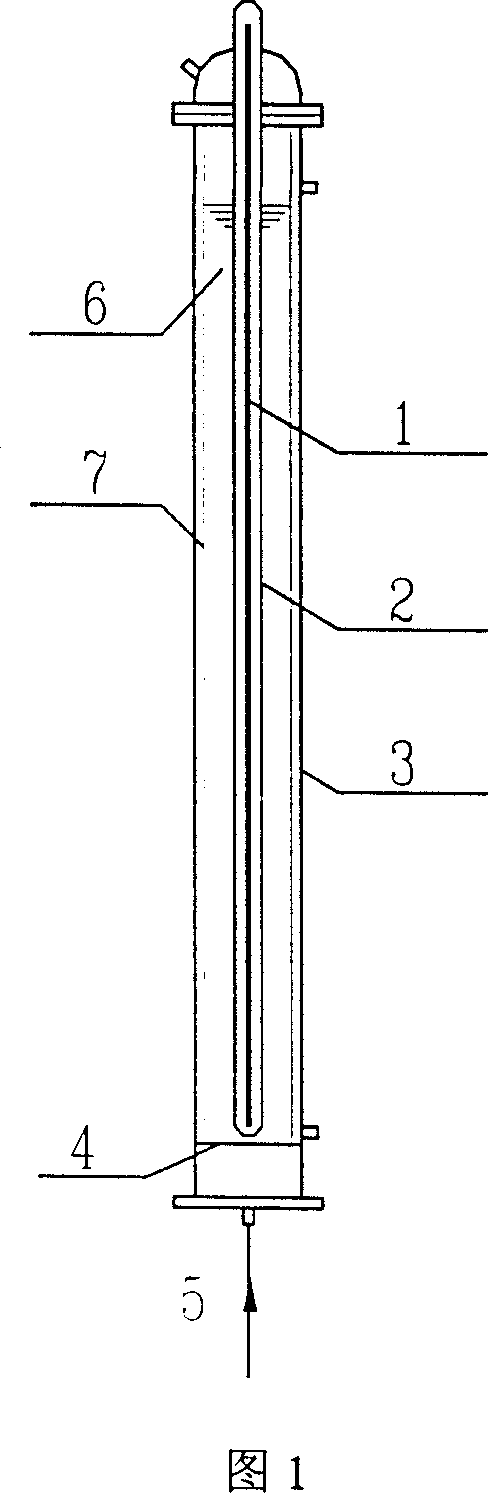
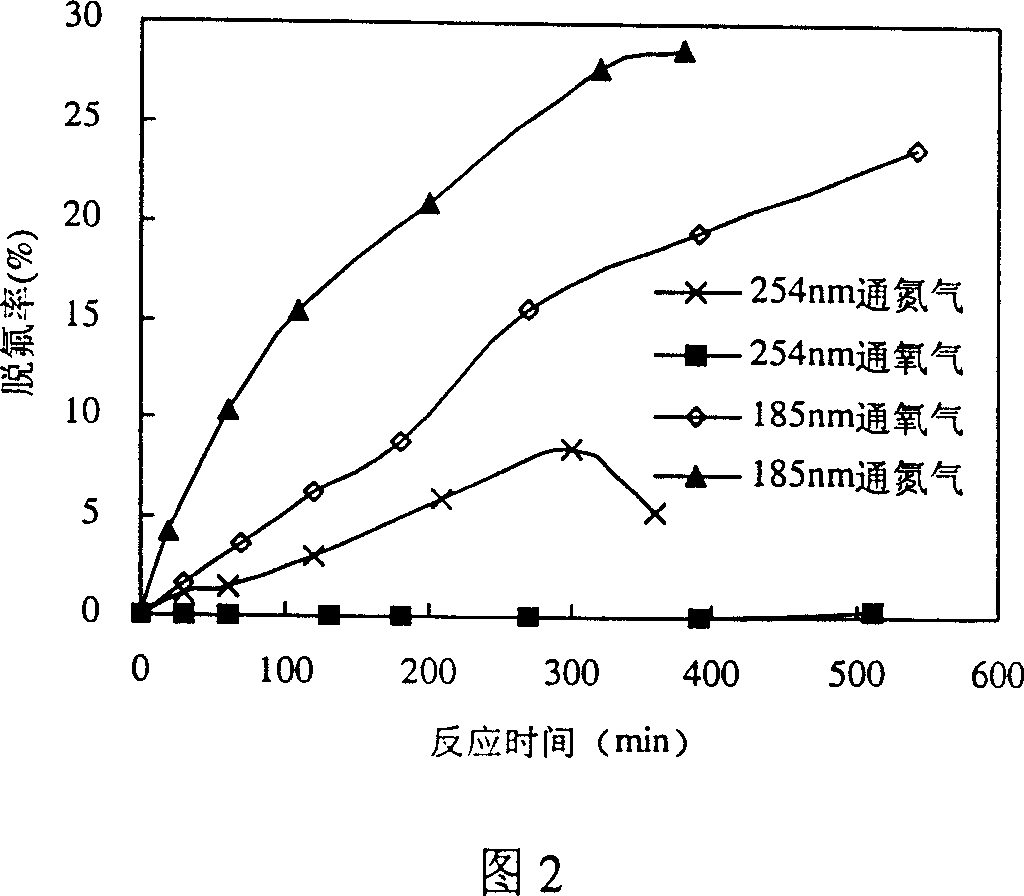
[0026]As shown in Figure 1, 800mL containing the PFOA aqueous solution of 15mg / L is put into reactor 3, and nitrogen is passed into reactor 3 from bottom gas distribution plate 4, and under the irradiation of 15W emission 185nm vacuum ultraviolet ray lamp 1, PFOA desorbs The effect of fluorine decomposition is shown in Figure 2. The defluorination rate is calculated according to the fluoride ion concentration in the water during the reaction, and the fluoride ion concentration in the water is measured by fluorine reagent spectrophotometry. Under the situation of feeding nitrogen under 185nm vacuum ultraviolet irradiation (indicated as "185nm passing nitrogen" in Fig. 2), the concentration of fluoride ions in the reaction solution increases with the reaction time, and the defluorination rate of PFOA reaches 28.7% after 380 minutes of reaction, the average Of the 15 fluori...
Embodiment 2
[0027] Embodiment 2: the vacuum ultraviolet ray reduction decomposition of perfluorooctane sulfonate (PFOS)
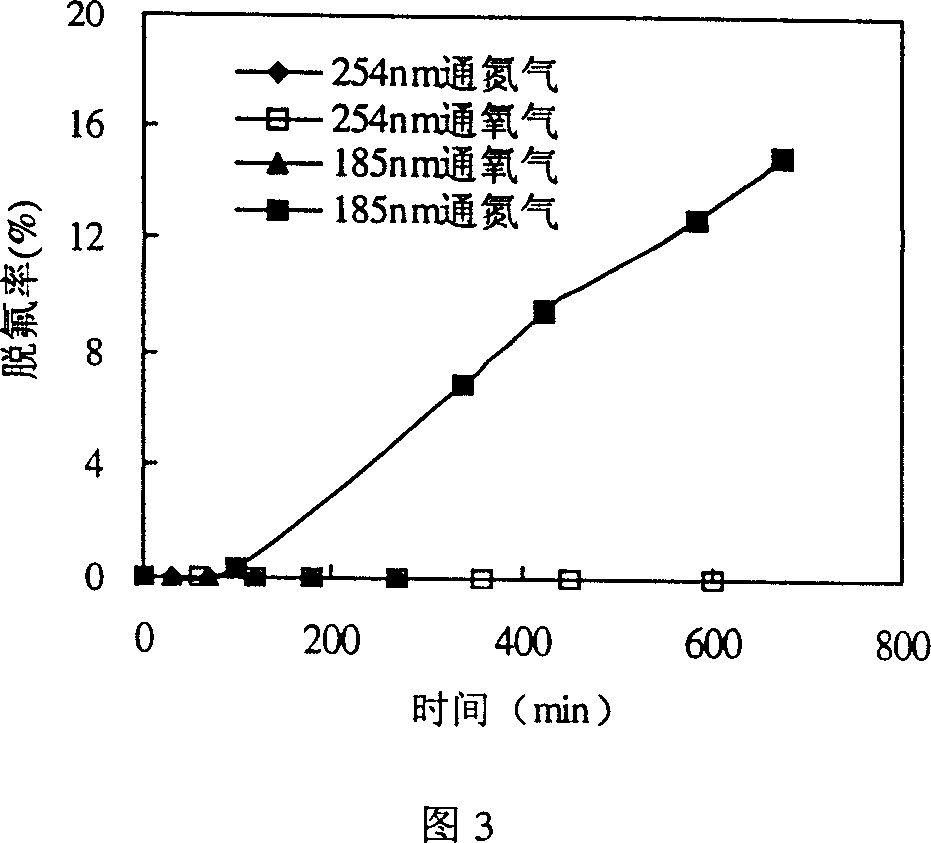
[0028] As shown in Figure 1, 800mL of PFOS aqueous solution containing 20mg / L is put into the reactor 3, and nitrogen gas is passed into the reactor 3 from the gas distribution plate 4 at the bottom. The effect of decomposition is shown in Figure 3. The fluoride ion concentration in the solution is measured by fluorine reagent spectrophotometry. The fluoride ion concentration in the reaction solution increases with the reaction time. After 675 minutes of reaction, the defluorination rate of PFOS reaches 15%. On average, PFOS molecules 2.55 fluorines of 17 fluorines (F) contained in have come off in the solution. In the case of 185nm vacuum ultraviolet radiation and oxygen, PFOS does not defluorinate and decompose; under 254nm radiation, no matter whether oxygen or nitrogen is introduced, it does not defluorinate and decompose.
Embodiment 3
[0029] Example 3: Catalytic defluorination and decomposition of perfluorooctanoic acid by vacuum ultraviolet light
[0030] As shown in Figure 1, 800mL of PFOA aqueous solution containing 19mg / L is put into reactor 3, nitrogen gas is passed into reactor 3 from the bottom gas distribution plate, and semiconductor photocatalyst LiNbO is added at the same time. 3 , the concentration in its reaction solution is 0.1g / L, under the irradiation of 15W emission 185nm vacuum ultraviolet ray lamp 1, the effect of PFOA defluorination decomposition is shown in Figure 4. Measure the concentration of fluoride ion in the reaction solution with fluorine reagent spectrophotometry, the concentration of fluoride ion in the reaction solution increases with the reaction time, the defluorination rate of PFOA reaches 23.5% after reacting for 210 minutes, on average, 15% contained in the PFOA molecule A fluorine (F) has 3.5 fluorine dropped into the solution. And correspondingly, if LiNbO is not adde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com