Glucagon-like peptide-2 and its therapeutic use
A technology of GLP-2 and aspartic acid, applied in the direction of glucagon, hormone peptides, specific peptides, etc., can solve the problems of little understanding of the physiological role of glucagon-like peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
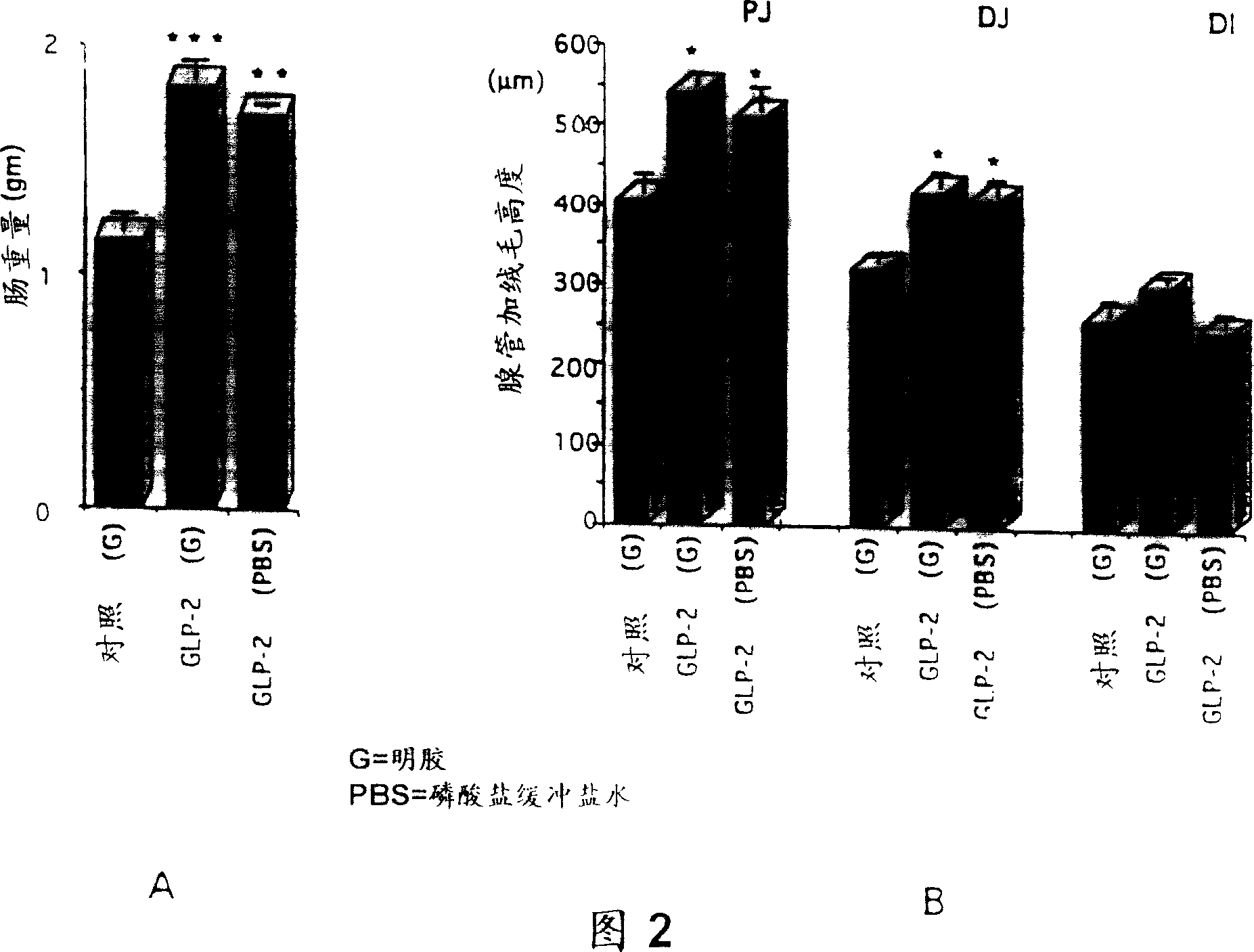
[0100] In the first experiment to study the effect of glucagon on the growth of the small intestine, two groups of mice (8 weeks old, CD1 female, obtained from Charles River Laboratories), 6 mice in each group, were treated as follows. Each mouse was subcutaneously injected with 0.5 cc of a 16% gelatin solution containing 41.5 µg of peptide every 12 hours for 10 consecutive days. Glucagon (rat) is easily soluble in 10ml of water. Control mice were injected with only 0.5cc of 16% gelatin solution without peptide every 12 hours. The mice were fed with standard rat chow, and the mice were given free access to food and water, and the mice were withdrawn from food and only given water 12 hours before sacrifice. For weighing the small intestine, the entire small intestine was dissected, followed by removal of the stomach (proximal) and appendix / cecum / large intestine (distal). The remaining small intestine was washed with saline to remove feces and weighed, with the following resul...
example 2
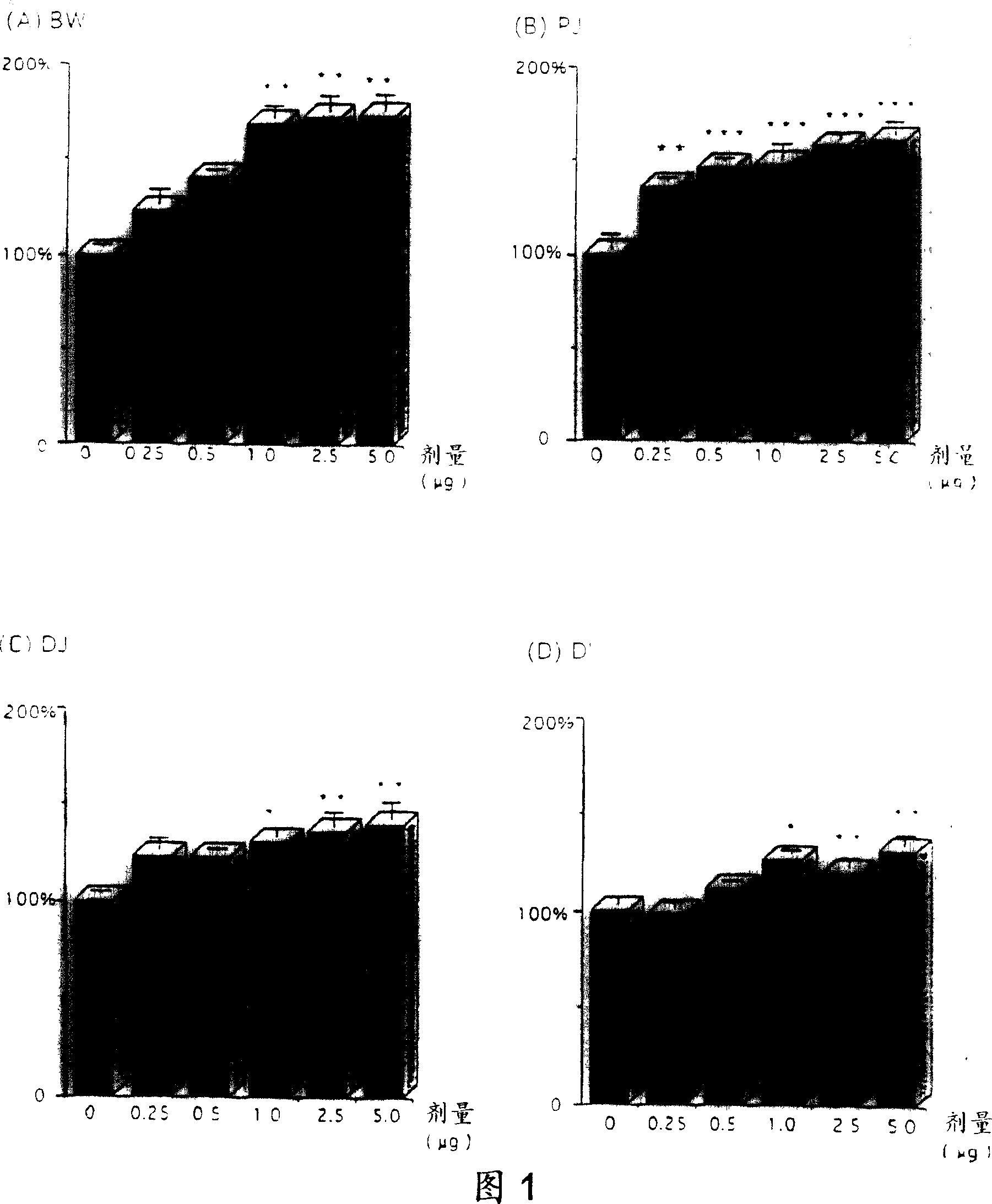
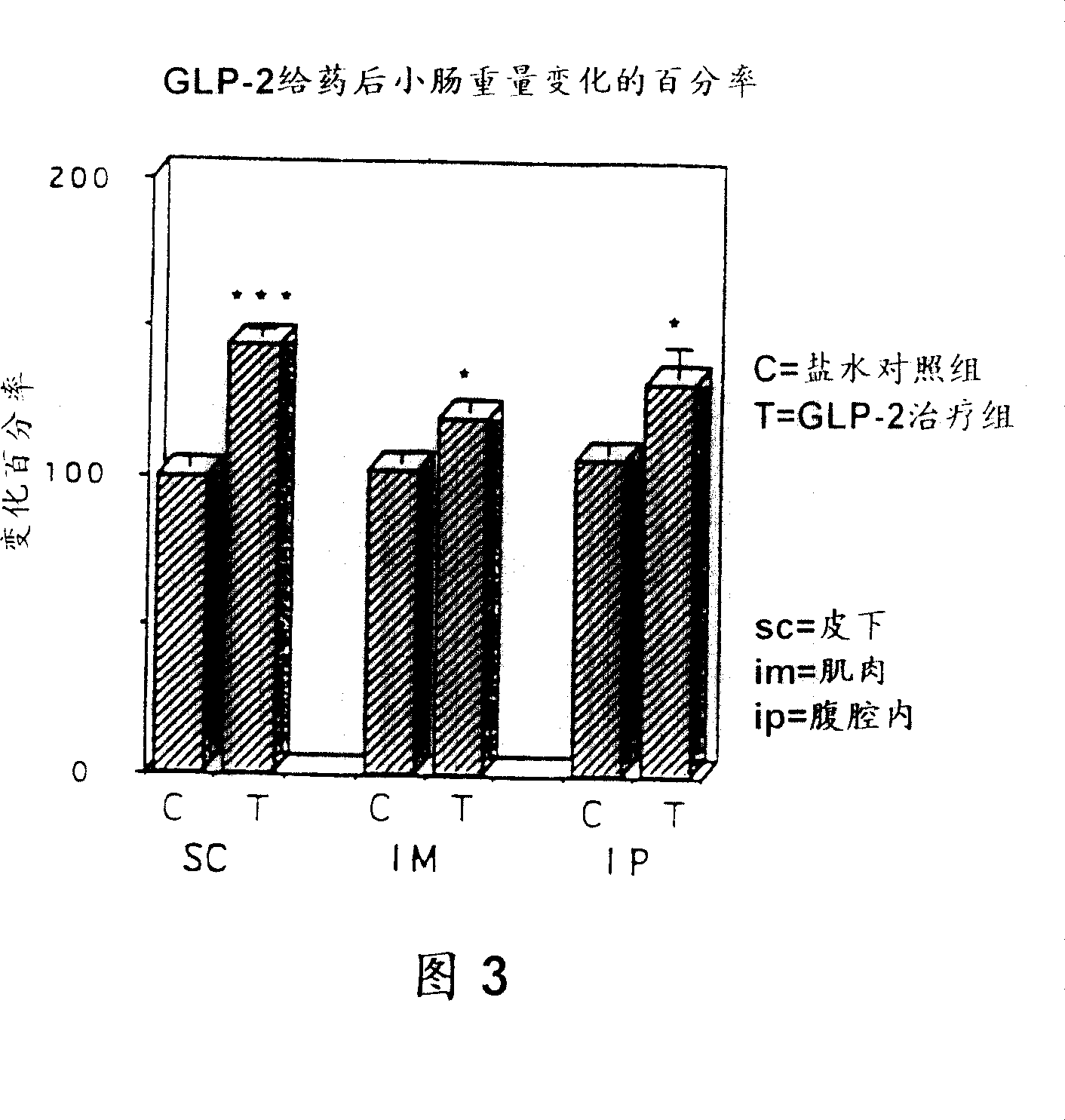
[0109] The effect of the GLP-2 peptide on the growth of the small intestine was further studied, especially to evaluate the relationship between the tissue response and the dose, time, route and frequency of administration, type of preparation, and sex and age of the subjects. The detection of these effects was reflected not only by an increase in the weight of the small intestine, but also by an increase in the height of the glandular ducts and villi.
[0110] For these purposes, rat GLP-2 was prepared as described in Example 3. This rat GLP-2 was formulated in phosphate buffered saline or as a depot formulation containing gelatin. The method of preparing GLP-2 peptide with phosphate buffered saline is as follows: first prepare 10×PBS stock solution, that is, weigh 80g NaCl (BDH ACS 783), 2g KCl (BDH ACS 645), 11.5g NaCl 2 HPO 4 (Anachemia AC-8460) and 2g KH 2 PO 4 (Malinckrodt AR7100), these reagents were dissolved in sterile distilled water in a total volume of 1 liter....
example 3
[0127] Given the results observed with rat GLP-2 in test mice, it was hypothesized that various vertebrate rat GLP-2 homologues and analogs could also mediate enterotrophic effects. For this purpose, various GLP-2 and GLP-2 analogs were synthesized and evaluated for their activity, as described below.
[0128] Solid-phase peptide synthesis (SPPS) was performed manually in 300 milliliter (ml) vessels at the 3 millimole (mmole) scale using 6 grams (g) of chloromethyl (Merrifield) resin (for C-terminal free acid peptide), 0.5 milliequivalents (meq) per gram of substitution. Amino acids are protected at the amino terminus with a tert-butoxycarbonyl (tBoc) group. Amino acid side chains are protected using benzyl (Bz, for serine and threonine), benzyloxymethyl (BOM, for histidine), 2-bromobenzyloxycarbonyl (2-BrZ, for tyrosine ), 2-chlorobenzyloxycarbonyl (2-ClZ, for lysine), cyclohexyl (cHex, for aspartic acid and glutamic acid), and tosylate (Ts, for arginine ) side chain prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com