Preparation process of p-bromofluoro benzene
A technology of bromofluorobenzene and fluorobenzene, which is applied in the field of preparation of binary halogenated aromatics, can solve the problem of high energy consumption and achieve the effect of simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
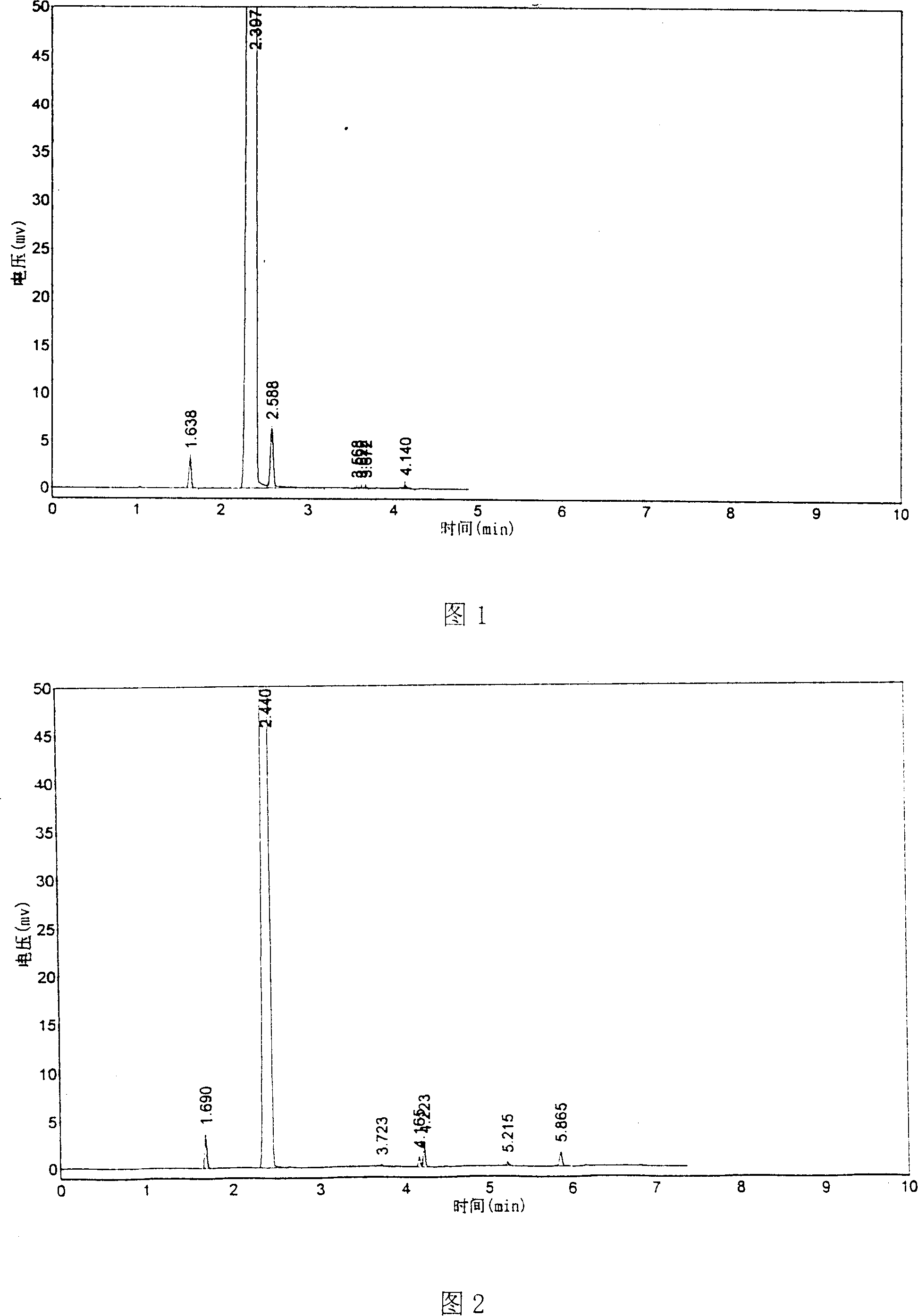
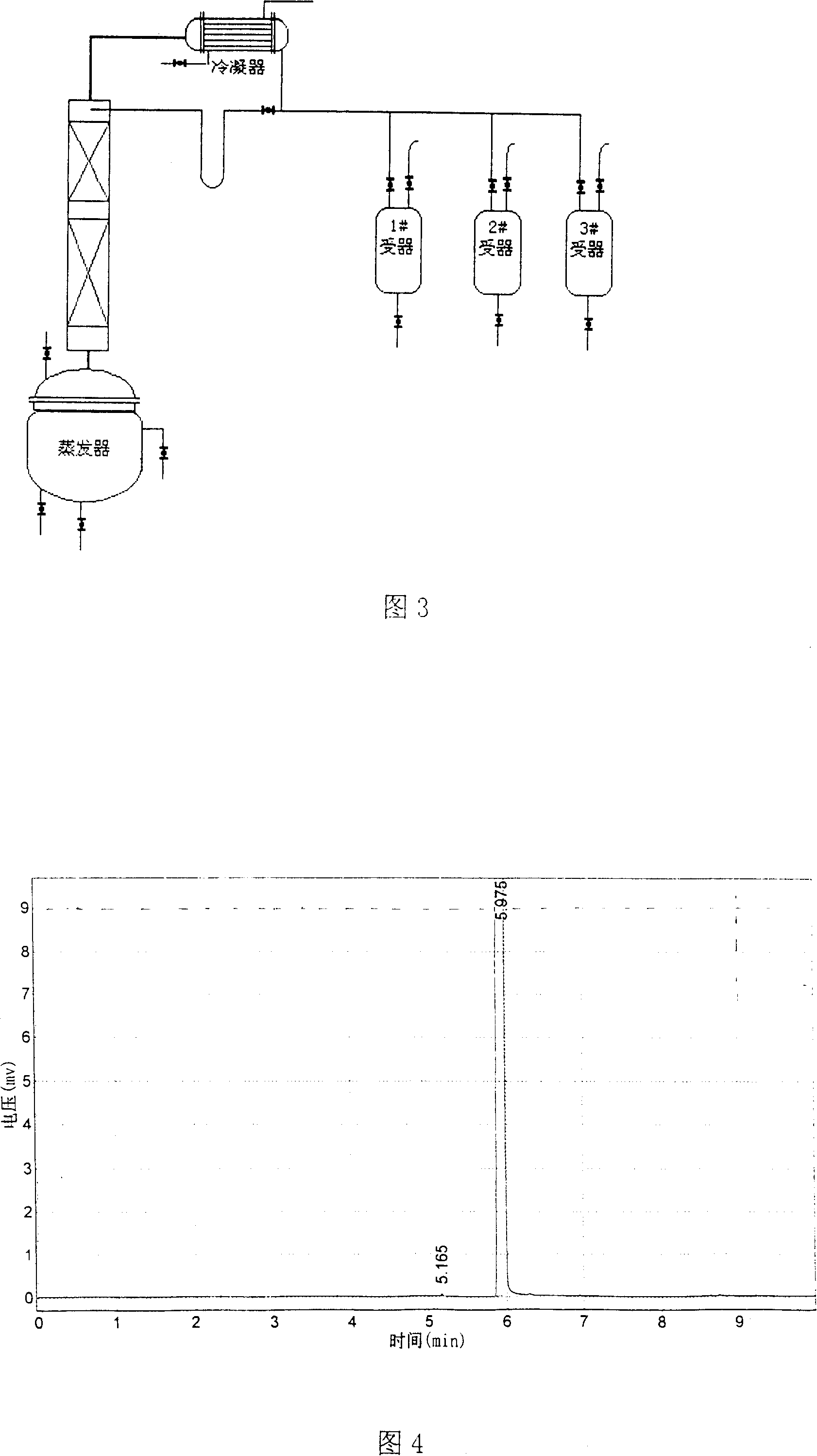
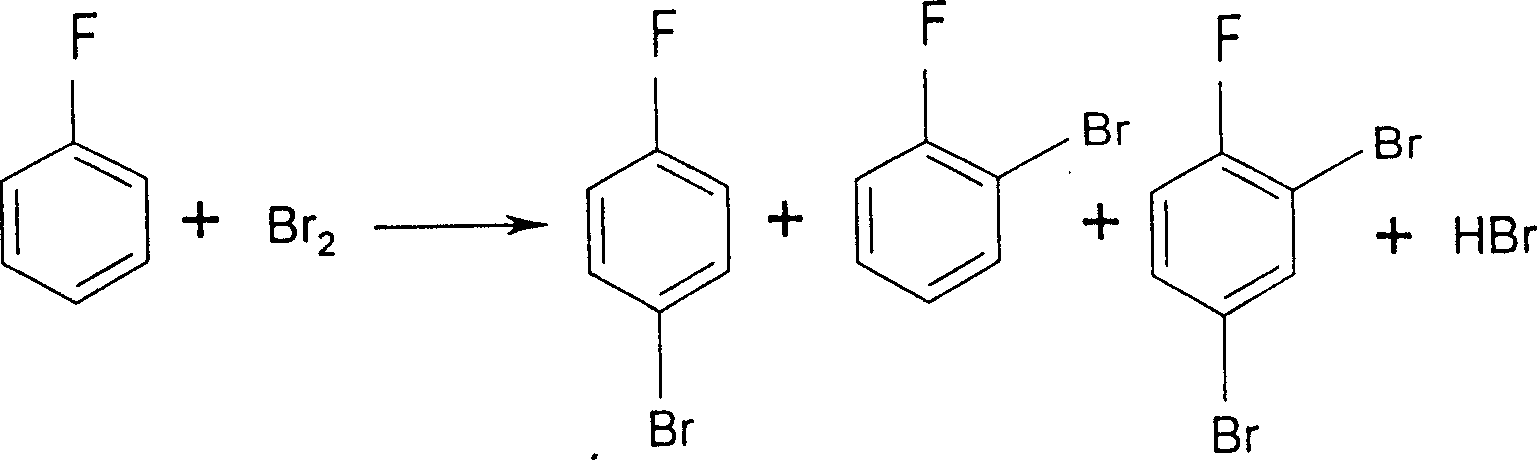
[0039] (Embodiment 1) In the five-necked glass reaction flask that communicates with the atmosphere with electric stirrer, thermometer, balance funnel, vent tube and reflux condenser of 1000 milliliters, add 500 grams of liquid fluorobenzene earlier, and make balance funnel and The outlet of the ventilation pipe is located under the liquid level of fluorobenzene; use a cold water bath to control the reaction temperature at 2±2°C; while stirring, add 2 grams of iron powder as a catalyst into the reaction flask, and add it into the reaction flask through the balance funnel 23 grams of liquid bromine make the material in the reaction bottle begin to carry out the initiating reaction of bromination reaction, and liquid bromine reacts with fluorobenzene to generate hydrogen bromide and p-bromofluorobenzene, and the by-product is o-bromofluorobenzene; the initiating reaction is carried out after 15 minutes , start to pass chlorine gas into the reaction system in the reaction flask th...
Embodiment 2
[0041]The rest are the same as in Example 1, except that the iron powder added as a catalyst in the bromination reaction is 1 gram, and the temperature of the bromination reaction is controlled at 15±2° C. After the reaction, 868 grams of finished product were obtained, and the yield of fluorobenzene was 93%. The contents of relevant components are shown in Table 1.
Embodiment 3
[0043] The rest are the same as in Example 1, except that the iron powder added as a catalyst in the bromination reaction is 0.5 g, and the reaction temperature of the bromination reaction is controlled at 25±2° C. The 858 grams of finished products obtained after the reaction have a fluorobenzene yield of 91.5%, and the contents of relevant components are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com